
DRS Dysphagia Digest 2017: Second Edition from the March 1-4, 2017 annual meeting of the Dysphagia Research Society. Held in Portland, OR. DRS celebrated its 25th anniversary!
DRS Dysphagia Digest
Thank you for reading the first installment of my DRS Digest 2017. Let’s check out 5 more highlights from the 2017 Annual Meeting and Post-Graduate Course of the Dysphagia Research Society. The majority of this information was from the post-graduate course on March 1, 2017. This additional day before the annual meeting has always been well worth it with directly clinically applicable information.
See you next year in Baltimore, Maryland for the 26th annual meeting on March 15-17th, with the post-graduate course on March 14, 2018.
1. Breathing and Swallowing Coordination
David Paydarfar, MD, from the University of Massachusetts Medical School in Worcester, presented on “Neurophysiologic Interactions Between Eating and Breathing.” Safe swallowing requires a specific pattern of breathing to be maintained. The central pattern generator (CPG) for our rhythmic breathing is in the brainstem, mediated by the Pre-Bötzinger Complex in the ventrolateral medulla. There is a primitive interaction between the two important central pattern generators for swallowing and breathing. Every swallow resets the phase of respiratory rhythm.
Our swallow is timed interrupting the exhalation phase with the period of swallow apnea. The food or liquid passes through the pharynx, finely coordinated with this period of not breathing. A normal healthy adult can maintain the period of apnea even with a large 100cc fluid bolus. The person will be vulnerable to aspiration if the swallow happens outside of this period. Even if the swallow is started later in the exhalation phase, the person may inhale before the bolus is fully cleared through the upper esophagus.
Problems with swallowing occur when there is/are:
- Weak reset of the respiratory rhythm
- Mistimings
- Poor control of respiration or disorders of respiratory control
- Swallow delays or any delays in bolus clearance
- Rapid respiratory rate, shortening the exhalation phases
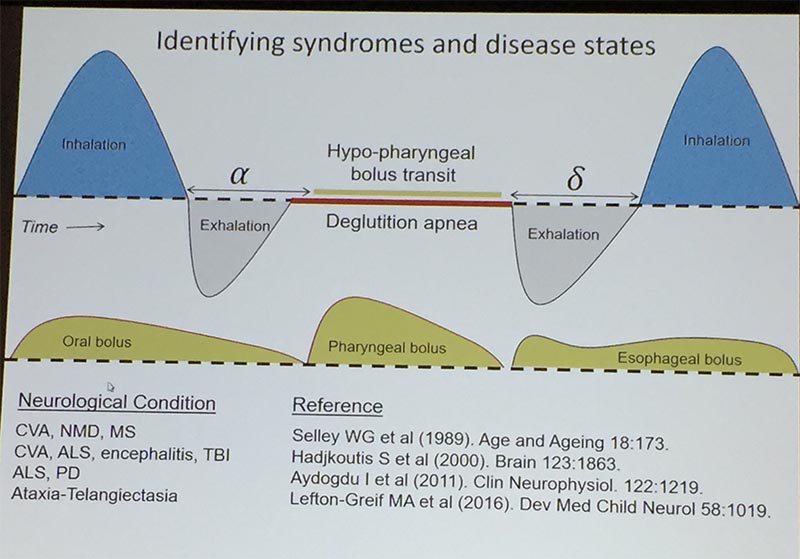
This picture was adapted by Dr Paydarfar from: Paydarfar, D., Gilbert, R.J., Poppel, C.S. & Nassab, P.F. (1995) Respiratory phase resetting and airflow changes induced by swallowing in humans. Journal of Physiology, 483, 273-288. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1157888/. Please see his suggested references at the bottom regarding how these disorders and diseases can further impact this delicate and complex coordination.
Paydarfar also referred to research published in the following article regarding the need for sensory feedback to facilitate adequate laryngeal closure and airway protection during swallowing:
2. Breathing and Swallowing Coordination with COPD
Dr Bonnie Martin-Harris, PhD, CCC-SLP, BCS-S, ASHA Fellow, from Northwestern University, discussed the physiological burden of breathing and swallowing on patients with severe COPD, which can lead to cachexia (i.e., wasting syndrome of significant weight loss and muscle atrophy). (Her session was titled: “Obstructive Pulmonary Disease – A Cross System Model of Impaired Airway Protection“).
She reiterated that swallowing occurs within the respiratory pause (aka, swallow apnea or deglutition apnea) during the expiratory phase. The swallow occurs at low-mid lung volumes within a relatively narrow range of quiet-restful breathing (no where near our total lung capacity). Therefore, telling people to take a deep breath in, swallow and then exhale may be counterproductive. The larynx is tethered in a downward position at the end of a deep inhalation. Additionally, per Martin-Harris, when we take a deep breath, the vocal cords are in an abducted (open) position and the pharyngeal airspace may be wider. The increased strain on the lungs in people with COPD may cause an even stronger downward traction on the larynx.
She noted that dysphagia in this population is “just so under-reported.” Many times there are no overt signs of aspiration bedside. The patients have poor sensation and awareness of their swallowing problems; therefore, an instrumental evaluation is critical.
Deficits that Martin-Harris noted that may be seen on the videofluoroscopic swallow study (modified barium swallow study) are:
- Slow/labored mastication, channeling food back to the pharynx while still breathing and chewing.
- Slow/discoordinated movements
- Head of the bolus deep into the pharynx before the swallow (maintaining breathing longer, while triggering the swallow later)
- Oral and pharyngeal residue
- Decreased laryngeal elevation and decreased laryngeal vestibule closure
- Early or late laryngeal vestibule closure, likely depending on how the person is compensating.
- Pharyngeal atrophy (may see very little tissue up against the cervical spine in the lateral view and pharyngeal out-pouching in the A-P view)
- Random, inconsistent penetration and aspiration (airway invasion) due to difficulty coordinating breathing and swallowing.
Please see Martin-Harris, et al. (2015) for her article titled: Respiratory-Swallow Training in Patients with Head and Neck Cancer. I look forward to Dr Martin-Harris’s continued work in Respiratory-Swallow Training (RST) and dysphagia therapy program development on how to train patients to recalibrate their respiratory patterning.
3. Eating and Swallowing Solid Food is Different from Drinking
Jeffery B. Palmer, MD, from Johns Hopkins University and prior DRS president from 2003-2004, was given the honor of presenting at the Dodds-Donner Lecture titled: “Eating and Swallowing Solid Food: A Physiological Approach.” He and colleagues have created The Process Model, which shows precise coordination and linkage of the tongue, jaw, hyoid, and soft palate. This model “is a validated physiological model for eating and swallowing solid food,” per Palmer.
“Eating and swallowing are highly complex and interdependent behaviors. (Read more about how when we assess patients for dysphagia, we look at more than just the swallow.)
Here are some key points of Palmer’s lecture:
- Eating is different than drinking
- Digestion begins with mastication, as food mixes with saliva.
- Strong linkages of the anterior tongue to the jaw and hyoid bone for the vertical movements.
- The concept that the posterior tongue is sealed to the soft palate is wrong for solids. While we are chewing, this area is open. The faucial pillars are open.
- Triturated food (aka, chewed food) squeezes back over multiple chewing cycles and can accumulate in the valleculae and even the pyriforms for up to 10 seconds. This is normal! See also Bonnie Martin-Harris’s
He reviewed his 3D kinematic studies of movement in stages 1 and 2 of food processing.
Stage 1 Transport: the rapid, almost simulaneous, “Pull-back” phase
- Jaw opens, food enters, jaw closes
- Tongue retracts, carrying food on its dorsal surface
- Jaw opens again to make room for the tongue to rotate to put food on the molar surfaces
- Rotational chewing pattern on the working side with a balancing action on the opposite side, but the food may be shifted from the working side to the balancing side
- Bolus is repositioned with every chewing cycle with jaw opening
- Actions of jaw opening and cheeks pushing on the food prevent biting of the cheeks and tongue
- “Soft” food may be compressed with the tongue and palate rather than the teeth
- Hyoid bobs with movements of the tongue and jaw
Stage 2 Transport: the “Squeeze back” phase
- Portions of food are propelled into the pharynx, while oral processing and mastication continue. Again, it is normal for food to collect in the valleculae and pyriforms for up to 10 seconds prior to the swallow.
- Tongue squeezes the food back along the palate
- The propulsion of the bolus into the pharynx is related to the jaw closing or when already approximated.
- The pharyngeal swallow is initiated as the jaw begins to open
So what about liquids?
While food may collect in the pyriforms for up to 10 seconds prior to the swallow, we know that even small cup sips of thin liquids may also drop to the hypopharynx and pyriforms in normal healthy adults before the swallow (see Martin-Harris, et al., 2007). The major difference is that the onset of the pharyngeal swallow subsequently occurs within milliseconds rather than up to 10 seconds. In the Martin-Harris, et al., 2007 study, more than 25% of the sample of 21 to 97 year olds initiated the swallow (as measured by the first hyoid burst/excursion for the swallow) when the bolus head was in the vallecular pit. However, 30% had the bolus in the hypopharynx (and one of these participants was 26 years old) and 12% of these normal healthy adults initiated the swallow with the bolus head in the pyriforms. All participants initiated apnea prior to the onset of this hyoid movement. Only 3 participants on this study had Penetration/Aspiration Scale scores of over 3 (out of 43 participants and over 2 trials). One patient had a PAS score over 3, and that was a 41 year old who scored a “6,” which is aspiration which was ejected. Can we call these “delays,” when the majority of the patients had no negative sequela? Do we really want to label our patients with the negative term of “delay,” when we may not be understanding the true range of normal? (Dr Ianessa Humbert asked this at our recent Critical Thinking in Dysphagia Management course. True, that is not the DRS meeting, but I had to throw that in there!)
4. Dysphagia in the Intensive Care Unit (ICU)
James Coyle, PhD, CCC-SLP, BCS-S, from the University of Pittsburgh, and Martin B. Brodsky, PhD, ScM, CCC-SLP, from Johns Hopkins University, conducted a session on the Evaluation and Treatment of Dysphagia in the ICU during the post-graduate course.
Dr Coyle repeatedly noted that the person’s underlying or primary disease processes may be key causes of the dysphagia, only exacerbated by the acute ICU factors, such as:
- Trauma to the oropharynx and larynx (e.g., from a large endotracheal tube, due to emergent intubation in the field, prolonged intubation and multiple extubations/re-intubations)
- Impaired pharyngeal and laryngeal sensation
- Impaired cognition and alertness (medications, sedation, neuromuscular blockade)
- Respiratory failure / Respiratory swallow incoordination
- Acute neuromuscular impairments
- Gastroesophageal reflux
What other patient factors do we have to consider?
- Baseline functional status
- Underlining diseases/conditions (e.g., congestive heart failure)
- Sepsis
- Age
- Critical illness, multiorgan failure
- Surgery (e.g., perioperative TEE)
- Long ICU and hospital length of stay
Regarding these factors above, Coyle and Brodsky referred to the following articles:
Macht et al 2013 Macht, M., Wimbish, T., Bodine, C. & Moss, M. (2013). ICU-acquired swallowing disorders. Crit Care Med, 41(10), 2396-2405. https://www.ncbi.nlm.nih.gov/pubmed/23939361
Skoretz, S.A., Flowers, H.L. & Martino, R. (2010). The incidence of dysphagia following endotracheal intubation: A systematic review. Chest, 137(3), 665-673. https://www.ncbi.nlm.nih.gov/pubmed/20202948
Dr Martin Brodsky discussed this systematic review. The 14 articles that were selected for review all had multiple shortcomings. For example in 8 out of 14 articles, dysphagia was defined as aspiration only. An instrumental evaluation was only completed in only 3 out of the 14 studies, and only after failing a swallow screen. One study defined dysphagia only by a swallowing latency.
Skoretz, S.A., Yau, T.M., Ivanov, J., Granton, J.T., Martino, R. (2014). Dysphagia and associated risk factors following extubation in cardiovascular surgical patients. Dysphagia, 29(6), 647-654. https://www.ncbi.nlm.nih.gov/pubmed/25119447
Endotracheal tube size
Potential risks from endotracheal tubes are so multifactoral and variable depending on the patient, and this is not the sole focus of this section (Read the section on “How and When to Evaluate Swallowing in Post-extubation ICU Patients” in this blog). However, I was shocked at the measurements Dr Coyle shared of the typical size of the human trachea. A study by Randestad, Lindholm & Fabian (2000) found that the cross-sections of tracheas varied, but the smallest inner diameter found in women was 9.9mm and 12mm in men. Coyle cautioned that the typical #8 endotracheal tube has an OUTER diameter of 9.6mm. The typical #8 tracheostomy tube has an outer diameter of 11.3. It is important to check the outer diameter of any of the tubes placed in your patient.
Number of ventilation days
Coyle highlighted the risks that are posed just with the number of ventilation days. In a study regarding post-extubation dysphagia in 270 trauma patients by Kwok, Davis, Cagle, Sue & Kaups, 2013, the incidence of oropharyngeal dysphagia was 42%. The number of ventilation days was the strongest predictor of dysphagia. There was an added 25% risk for each day on the ventilator. The study recommended when trauma patients are on a ventilator for greater than 2 days, swallowing should be assessed upon extubation.
High-flow nasal cannula and swallowing
Coyle noted that there is no conclusive evidence yet regarding if high-flow nasal cannulas (HFNC) increase aspiration risk, but some patients may not be able to handle the pressures that are at about half of those that are deliviered by adult CPAP systems. He recommended an article by Ward (2013) titled: High-flow oxygen administration by nasal cannula for adult and perinatal patients. The Ward article reviewed a study where the mean pharyngeal pressures with the mouth closed were: 8.7 cm H20 at 60 liters/minute and 7.2 cm H20 at 40 liters/minute. The article’s summary noted:
“There are safety concerns for developing unintended high airway pressure.”
However, these safety concerns related to eating and swallowing while wearing HFNC were not delineated. In fact, the Ward article concluded that HFNC may be more advantageous than a face mask for speaking, eating, drinking, and expectorating pulmonary secretions. This area needs more research. As dysphagia clinicians, we have to ask: what are the that are requiring such high oxygen demands, and how stable are they to be eating and drinking? For example, what is the patient’s respiratory rate at rest? If it is 20 breaths per minute, that is fine. If it is greater than 30, then maybe the person is not stable enough to be consuming meals. When the person starts eating and drinking, is there an increased work of breathing and an increased respiratory rate that could impair the normal respiratory swallowing coordination? (Read more on critical values, like respiratory rate.)
Pulse oximetry & detecting aspiration
Coyle pointed out that SpO2 (blood oxygen saturation level) does not detect aspiration. (Read more in the critical values blog above and in this blog highlighting Dr Steven Leder’s work).
This was also the focus of a paper presentation at #DRS2017 by Britton, D., Lederle, A., Ennis, S.K., Bandit, J.O., Quinn, C. & Graville, D.J. titled: Utility of Pulse Oximetry in Detecting Aspiration – Evidence Based Systematic Review. They concluded:
“Current evidence does not support the use of pulse oximetry to detect aspiration.”
5. Dysphagia Treatment in the ICU
“Treatment to improve swallowing needs to improve,” said Brodsky.
Per Brodsky, SLPs need to increase their presence in the ICU. He noted how there are no critical pathways for rehabilitation in the ICU (See Brodsky, et al., 2014), and there is limited awareness of the potential for long-term effects of a prolonged ICU stay. Our field needs to increase the awareness of dysphagia risks in critical care. However, existing SLP training programs may have limited exposure to the complexity of critical care.
Large muscle groups
Brodsky noted that “we have known for a while that large muscle groups are weakened during the course of an ICU stay.” In a British study by Puthucheary, et al., 2013, 63 critically ill patients were studied for skeletal muscle wasting during ICU stays of 7 days or longer. One of their markers for muscle mass was to measure the cross-sectional area of the rectus femoris using B-mode ultrasonography. This area decreased significantly by day three! There was a 12.5% decline from the 1st day to day 7, and a 17.7% decrease by day 10. The table in the article shows a striking linear drop over the 10 days. Multiorgan failure correlated with this change in the rectus femoris, as opposed to a single organ failure. A logistic multivariable regression analysis was done to determine clinical correlates. Age was one of the variables associated with a greater than 10% loss in rectus femoris area. Age showed an odds ratio of 1.05 per year; therefore, an ICU patient is 1.05 times more likely to have muscle wasting per every year of age. That adds up for our 80-90 year olds versus when compared to the 50-60 year olds.
Brodsky noted that ICUs are tending toward lighter sedation protocols, per the Society of Critical Care Medicine (e.g., holding off on Benzos and reducing the use of neuromuscular blockades – See more on medications and dysphagia). Have you seen how our physical therapy colleagues are mobilizing patients while still on the ventilator?
Small muscle groups
Brodsky is interested in bringing this level of mobilization to the small muscles of swallowing and comments:
“One of the reasons for weakness, seemingly no matter where it is localized in the body, is due to the duration of inactivity.”
Su, et al. (2015) studied tongue weakness and sensory disturbances in 30 previously intubated patients (mean of 7.8 days intubated) versus 36 controls. Measures of tongue strength were taken with the IOPI (Iowa Oral Performance Instrument) after extubation at 48 hours, 7 days and 14 days. Tongue strength in the previously intubated patients was 38% less than the controls on average at 48 hours post-extubation. These patients made progress by day 14, but still did not catch up with controls. Sensation was also significantly reduced at 48 hours and at 7 days, but they did catch up with controls by 14 days post-extubation.
Maybe we should be moving the endotracheal tube to the side and providing patients with sensorimotor stimulation and exercise, per Brodsky. This would be an excellent study with patients on the ventilator who are already able to mobilize with physical therapy.
The small muscle groups involved with swallowing were the focus of a recently published 5-year longitudinal outcomes study by Dr Brodsky and colleagues (See Brodsky, et al., 2017). As noted in the study’s rational:
“Nearly 60% of patients who are intubated in intensive care units (ICUs) experience dysphagia after extubation, and approximately 50% of them aspirate. Little is known about dysphagia recovery time after patients are discharged from the hospital.”
This study followed 115 patients for up to 60 months after mechanical ventilation and found:
- One-third of the patients have dysphagia that persists after discharge, and
- 23% have dysphagia remaining after 6 months.
Interestingly, longer lengths of stay in the ICU caused more persistent dysphagia. Brodsky noted during his DRS session: “each 1 day increase in ICU length of stay is independently associated with a 4% reduction in the probability of recovery.”
In discussing this research with Dr Martin B. Brodsky recently, he had these summaries:
“For those with symptoms of dysphagia post-extubation, these are likely to continue for a long time, typically 3-6 months after hospital discharge, but lasting as long as several years. The good think is that ALL of the patients did recover from these symptoms.”
He further commented that the editorial by Kruser & Prescott (2017): Dysphagia after Acute Respiratory Distress Syndrome summarized this issue wonderfully with:
“Existing evidence demonstrates that dysphagia after ARDS is common, persistent, clinically important, and treatable. Yet, it remains underdiagnosed and overlooked. Brodsky and colleagues add to the compelling body of evidence that suggests screening for dysphagia after extubation should become part of our routine clinical practice for patients surviving critical illness.”
Kruser & Prescott advocated for the dysphagia to be considered an important feature in this Post-Intensive Care Syndrome.
Early Intervention starts with the instrumental
Regarding the implementation of treatment early on, Brodsky commented on a national survey by Macht, et al. (2012) of 1,966 SLPs who regularly evaluate and treat patients for post-extubation dysphagia. Many SLPs commented that treatment focuses primarily on dietary texture modification (76%) and compensatory strategies (86%). Only 24% of SLPs stated that they focus on interventions to improve or rehabilitate the actual swallowing function.
Brodsky advocated for SLPs to start therapy immediately. For example, treatment can start with the instrumental evaluation. Of course during the instrumental evaluation, we identify the underlying impairments, judge severity, determine the appropriate diet/liquids and the route and manner of intake, and make appropriate referrals. However, we also start focused treatment strategies during the VFSS or FEES. Then, in the report, our recommendations should not only include diet modifications, but also what worked and what exercise program could be matched to the structural and physiological findings. That way, future clinicians can focus treatment and have a rationale from your report to support the exercises they choose. Keep in mind, diet modifications and compensatory strategies and maneuvers are like crutches. They are important in the early days of recovery, but they are not rehabilitative. Additionally, if you think someone needs a strategy like the Mendelsohn Maneuver or an effortful swallow, which can be rehabilitative and strengthening, then these must be done under fluoroscopy or manometry first. This is need to make sure the behaviors are not maladaptive or ineffective. Use the live video image as biofeedback to reinforce the given maneuver, strategy or exercise. (These concepts were mentioned by Brodsky at his talk, and are directly in line with what we are taught by Dr Ianessa Humbert and Dr Emily Plowman at the Critical Thinking in Dysphagia Management course – #CTDM).
Summary:
Dysphagia is under-reported,
under-diagnosed and overlooked.
In finishing up this blog of these 5 highlights from #DRS2017 (Annual Meeting & Post-Graduate Course of the Dysphagia Research Society), I was struck by the theme of under-diagnosis, particularly in patients with respiratory compromise (like COPD) and who are in critical care. This could be due to a lack of protocols in place in the ICU that lead to referrals for comprehensive dysphagia evaluations by Speech-Language Pathologists. It highlights the need for us to become strong advocates (click here for prior blog) for our field, for our staffing and equipment needs, and for our patients.
On the other hand, therapists have to be careful to not over-diagnose (therapy diagnosis, not medical diagnosis) once that patient is in our care. Dr Ianessa Humbert labels this a “confirmation bias.” (Humbert discussed this at the Critical Thinking in Dysphagia Management course.) Imagine: you have the patient in the fluoroscopy suite to find out what is wrong, so you may be unconsciously biased to find and report only the negatives, rather than what worked! This may lead to an over-diagnosis of issues like a “delay,” as discussed above in section #3 on eating is different than drinking.
Thanks for reading. Please feel free to comment or ask questions!


Dysphagia can be serious. Someone who cannot swallow safely may not be able to eat enough of the right foods to stay healthy or maintain an ideal weight.
Interesting topics. Additional knowledge that will be very useful in my current practice and topics to be discussed with our students
I consider this to be a great article! I have only one thought. How do you do it? Everything on this website is really good.