Take the RAD out of Radiation-Associated Dysphagia (RAD): What We Know About Acute through Late Effects of Chemoradiation
By Allison Bartholow, MS, MS, CCC-SLP & Karen Sheffler, MS, CCC-SLP, BCS-S of SwallowStudy.com
April is Head and Neck Cancer Awareness Month
See this link to the American Association for Cancer Research (AACR) for more information on Head and Neck Cancer Month.
Thank you to my guest writer, Allison Bartholow, for this article that will cover the acute, chronic, and late effects of chemotherapy and radiation treatments used to treat head and neck cancer. These side effects may cause difficulty swallowing (dysphagia), also called radiation-associated dysphagia (RAD). What can we do about that?
First, we need to understand the issue better. Let’s start with a story of a person’s struggle with a late onset of radiation-associated dysphagia (RAD).

April is Head and Neck Cancer Month. Time to learn about radiation-associated dysphagia (RAD) and how to evaluate and manage this and other toxicities from treatment for head and neck cancer.
Introduction to Radiation-Associated Dysphagia with a Case Study:
A 55-year-old male presented to the Swallowing Disorders Clinic with complaints of food getting stuck in his throat (i.e., globus sensation) and coughing with thin liquids. He noticed these persistent and worsening symptoms for the past six months. He reported an episode of aspiration pneumonia three months prior. He shared his history that 5 years ago, he had squamous cell carcinoma (cancer) of the right base of tongue and left tonsil. His treatment was extensive with chemotherapy and a full course of radiation to the neck, completed in 2019. (This treatment is called concurrent chemoradiation). Now, at the swallowing clinic, he received a comprehensive dysphagia evaluation by a speech-language pathologist (SLP) that included a Modified Barium Swallow Study (aka, videofluoroscopic swallow study). This showed a significant difficulty swallowing (dysphagia).
At this point, we will share detailed findings for our SLP and dysphagia specialist readers: His dysphagia was characterized as a moderate-severe oropharyngeal dysphagia. Deficits included: reduced base of tongue retraction with reduced tongue base to posterior pharyngeal wall contact, diminished pharyngeal stripping wave, reduced hyolaryngeal excursion (anterior and superior movement of the hyoid and larynx), incomplete laryngeal vestibule closure (lack of closure of the top of the airway), and reduced opening and distension of the upper esophageal sphincter. Due to these physiological deficits, he had safety (i.e., aspiration) and efficiency (i.e., residue) issues. The safety issues lead to silent aspiration when drinking thin liquids, and the efficiency issues lead to a significant collection of residue in valleculae and pyriform sinuses after the swallow with liquids and solids (about 50% of the bite/sip of food/liquid remained). Specifically, he had consistent penetration to the level of the vocal cords during the swallow, leading to consistent silent aspiration during the swallow. He also had silent aspiration on thin liquids after the swallow due to the residue that remained in his lower throat/hypopharynx spilling into the larynx (top of the airway). The deficits were mitigated (i.e., reduced, as risks may not be prevented) with the following safer swallowing strategies: right head turn/tuck when taking sips of thin liquids, multiple swallows to clear residue, and alternating liquids and solids (as the liquid wash helped to clear solid residue).
In a person-centered care approach, he was educated and trained by watching the video of his swallowing during and after the study. The SLP discussed options and relative risks. Based on this discussion, he agreed that the best option seemed to be to continue his current diet of regular solids and thin liquids, but to add the trained safer swallowing strategies. He demonstrated understanding as to why he benefited from these compensatory swallowing strategies. He also understood how he could try to reduce his risk of repeated aspiration pneumonias by improving his oral hygiene and continuing his daily walking and exercise regimen.
However, he had questions about why he was having such trouble swallowing now and not right after the chemotherapy and radiation. He recalled having such trouble with mouth sores and pain with swallowing right after his cancer treatment (i.e., from the acute chemoradiation toxicity or side effects), but then after a few months, he felt he was swallowing just fine. There were no sudden neurological changes that could explain this recent decline in swallowing as of 6 months ago. He spent so much money on fixing his teeth after radiation, so why is he having such trouble chewing and swallowing 5 years later? He was very upset and frustrated.
Question: What could be the cause (etiology) for this decline in swallow function 5 years after cancer treatment?
Answer: Late RAD — read on to learn more about radiation-associated dysphagia and the early and late effects.
This is a Public Health Issue
The difficulty swallowing (dysphagia) that is frequently associated with head and neck cancer and its treatments is a public health issue. It is called radiation-associated dysphagia or RAD. It is important to raise awareness among healthcare professionals and the public. With the rise in HPV-positive cancers of the head and neck (oropharyngeal cancers), there has been a change in demographics. Surviving any type of head and neck cancer at any age is challenging and a success story. However, rather than being 70-80+ years old, HPV-positive cancers often impact younger adults between the ages of 40 and 50 years of age (Young et al, 2015). Additionally, the likelihood of people surviving these initial cancers is very high. Therefore, these younger individuals will have another 40-50 years of living with the outcomes and side effects. It is critical for both the public and healthcare professionals to identify and reduce the negative side effects of head and neck cancer, especially as it relates to the individual’s quality of life, nutrition, hydration, livelihood, and overall health.
Toxicity from Head and Neck Cancer Treatments
This radiation-associated dysphagia is considered a toxicity that results from the treatment of the cancer. Let’s learn more about these therapies and their toxicities.
Head and neck cancer involves the anatomic regions of the nasal cavity, mouth/oral cavity, throat/pharynx, and/or the voice box/larynx, which can be treated with a combination of surgery, chemotherapy, and/or radiation therapy. Treatment “toxicities” or side effects occur as a result of chemotherapy and/or radiation therapy. These toxicities can happen:
- Right away or within the first few months (acute effects),
- After a few months to 5 years after the end of treatment (chronic effects), and/or
- Manifest after 5 years after a period of recovery without significant symptoms (late effects).
The management of toxicity effects is a common problem for people who are survivors of head and neck cancer. Toxicities because of cancer treatment are graded from Grade 1 to Grade 5 through the Common Terminology Criteria for Adverse Events, or CTCAE, according to the U.S. Department of Health and Human Services.
Common Terminology Criteria for Adverse Events (CTCAE), version 5 (U.S. Department of Health and Human Services, 2017)
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Mild | Moderate | Severe | Life-threatening consequences | Death related to Adverse Event (AE) |
| Asymptomatic or mild symptoms, clinical or diagnostic observation, intervention not indicated | Minimal, local, or non-invasive intervention | Medically significant but not immediately life threatening; hospitalization indicated; disabling | Urgent intervention indicated |
Acute Toxicity and Examples:
Acute toxicity effects are generally resolved within the first 3 months or 90 days post treatment with chemoradiation (Murphy & Gilbert, 2009). The following are side effects of chemoradiation or radiation alone.
- Dermatitis is skin irritation as a direct complication of radiotherapy alone, often observed within weeks of radiation initiation. It manifests as erythema, or redness and dry skin, which can progress to ulceration (Salvo et al, 2010). This side effect will generally resolve 2-4 weeks following the end of treatment. It is estimated that 85% of individuals will experience some degree of dermatitis (Salvo et al, 2010).
- Mucositis is inflammation secondary to tissue damage, which is most often seen in the oral cavity. The severity of mucositis can be related to low white blood cell count and the presence or extent of necrosis (Kearney & Cavanaugh, 2019). Mucositis is graded from Grade 0, without signs or symptoms, to Grade 4, with evidence of ulcers requiring alternative nutritional support, according to the World Health Organization. Side effects of mucositis include ulcers, pain, and lesions. Use of oral care protocols (i.e., oral infection control) and pain management can assist in improving symptoms (Kearney & Cavanagh, 2019). Mucositis can also enhance sensitivity, manifesting as a burning sensation, which is heightened with spicy, dry, or acidic foods (Murphy & Deng, 2015).
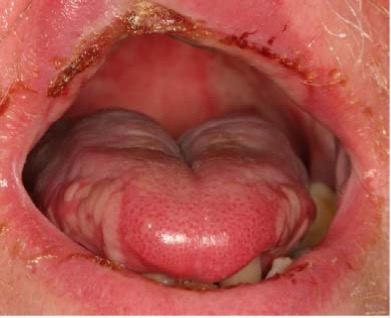
Example of oral mucositis (AAOM, 2015)
- Xerostomia is the lack of saliva production resulting in dry mouth. This increases the risk of oral infections, mucositis, oral pain, dysphagia, cuts/lesions in the oral cavity, and halitosis (bad breath). It is commonly seen in both the acute and chronic stages after radiation. Due to damage to the tissues of the salivary glands or injury to salivary ducts, saliva production is directly impacted. To manage the symptoms, try the following: meticulous oral infection control (see also this webinar), sugar-free candy and gum to stimulate saliva flow, oral moisturizing agents/artificial saliva substitutes, and humidification (Kearney & Cavanaugh, 2019).
- Taste changes are often observed during the acute stage of treatment, but they may also be present in the chronic stage. Symptoms may be present 6 months following the end of treatment but may continue up to 7 years later. The following taste changes may occur:
- Dysgeusia or taste alternations;
- Ageusia or lack of taste;
- Hypergeusia or hyperactive taste; and
- Taste phantoms.
- Dysphagia (difficulty swallowing) is commonly seen during the acute stage. Often seen is difficulty with mastication or chewing, odynophagia or pain, globus sensation, which is the sensation that food or drink are stuck in the throat. These symptoms may be caused by or exacerbated by the other issues of: taste changes, mucositis, and xerostomia. Additionally, pain when swallowing (aka, odynophagia), nausea/vomiting, reduced appetite, and fatigue also may impact overall intake.
Chronic and Late Toxicity and Examples:
The Chronic Toxicity effects are observed from 90 days to 5 years after radiation treatment, whereas the toxicity is labeled as Late if it is 5 years or more post-treatment (Murphy & Gilbert, 2009). The following examples are issues within these two time periods.
- Osteoradionecrosis (ORN) is a painful chronic or late effect causing necrosis (death of body cells in tissues or organs) of the bone because of:
- high dose radiation,
- purulence (formation of pus), or
- fistulas (abnormal connection between two cavities).
- It is graded on a scale of 1-4, where 4 is indicative of the need for surgical intervention. Most recently, Wong and colleagues (2017) reported 34 people (9.7%) of a sample size of 349 developed ORN with a median latency of 78 months (about 6 and a half years). Of these 34 people, 35% developed chronic dysphagia. Individuals with ORN are 4.7 times more likely to develop chronic dysphagia, with a majority presenting with Grade 4 ORN (Strojan et al, 2017).
- Trismus is the development of contractures in the jaw muscles, resulting in reduced mouth opening. It is theorized that fibrosis (i.e., abnormal healing response that damages tissues with increased collection of proteins) contributes heavily to the development of trismus. It is observed within the first 9 months post radiotherapy. There is a 24% chance of developing trismus per every additional 10 Gy applied to the pterygoid muscles. Functional impairments, such as difficulty opening the mouth and reduced ability to chew solids, have also been noted in individuals who received a total of 15 Gy to the pterygoid muscles (Strojan et al, 2017).
- With teeth, trismus = mouth opening is less than 35 mm (i.e., less than 35mm – about 1.38 in) to 1.4 inches (i.e., about width of two thin fingers) of opening in the front of the mouth between upper and lower teeth (Strojan et al, 2017).
- Without any teeth or dentures (edentulous), trismus = mouth opening is less than 40mm (about half the length of the long edge of a credit card) (Strojan et al, 2017).
- Gray or Gy is the unit that measures the absorbed dose of radiation and is used when describing the amount of radiation an area of the body absorbs in the context of radiation therapy (USNRC, 2021).
- Pterygoid muscles (lateral and medial) are heavily involved in mastication and are located in the inferior temporal region (Rathee & Jain, 2024).
- Fibrosis can be observed throughout the aerodigestive tract, which is all the organs and tissues of our respiratory and upper digestive tract (e.g., mouth, nose, tongue, throat, voice box/larynx including vocal cords, windpipe/trachea, and the food tube/esophagus). More on fibrosis later in this article.
- Ototoxicity is damage to the ear’s cochlear cells or to the eustachian tube. As a result of radiation to the eustachian tube, obstruction of the tube can occur due to edema or may contribute to increased otitis media with effusion (ear infections) (Upadhya et al, 2011). Maintaining a dose of less than 47 Gy (<47 Gy) helps prevent hearing loss (Murphy & Deng, 2015).
- Neck and shoulder dysfunction are often seen in individuals who have undergone neck dissection or surgical resection. It can often be seen as a chronic or late effect of radiation, due to radiation fibrosis syndrome (RFS). This leads to poor posture with evidence of:
- kyphosis or rounding of the upper back,
- loss of lordosis or natural curvature of the lower back or lumbar spine, and a
- forward head position (Stubblefield, 2011; Murphy & Deng, 2015).
- Systemic changes are reported as a chronic effect of radiotherapy. Based upon quality-of-life surveys and questionnaires, many survivors of head and neck cancer report fatigue and sleep disorders, which may be impacted by premorbid conditions (issues present before the cancer diagnosis) such as sleep apnea (Murphy & Deng, 2015). Another side effect is hypothyroidism, which may be a result of thyroid failure secondary to the high dose of radiation to the region. Ranta and colleagues (2022) reported 67 cases or 29% of people were diagnosed with hypothyroidism following head and neck cancer treatment. They reported a median latency to initiation of medication at 2.5 years. In other words, these individuals suffered from symptoms of hypothyroidism for 2 1/2 years before it was identified and treated fully. One more example of why this is a public health issue.
- Lymphedema is edema or swelling of tissues due to impaired tissue drainage. This is often experienced as a chronic effect of radiotherapy. Lymphedema can lead to tissue fibrosis. It can be external or internal. Depending on the extent of lymphedema, it can significantly contribute to the severity of the dysphagia (Kearney & Cavanagh, 2019). This is an important area for SLPs to collaborate across the team with certified lymphedema therapists (CLT), physicians, and physical therapists to holistically treat a person’s dysphagia. For more information, please see the library of the Dysphagia Research Society’s Institute for Education (DRSIE). As a DRS member, you have full access to the whole library. There is a talk on Lymphadema by Dr. Kate Hutcheson and Heather Starmer from July 2023. See also: https://www.ahns.info/survivorship_intro/topical_review-lymphedema/
- Cranial neuropathy and neuronal injuries can occur in acute through late stages. More on these in the Late RAD section below. Read more here.
- Dysphagia: the following summarizes the oropharyngeal and esophageal dysphagia side effects.
- Oropharyngeal dysphagia is having difficulty swallowing in the oral/mouth and pharyngeal/throat stages. Significant oropharyngeal dysphagia is often observed as a chronic radiation side effect in the 12 months after radiation. However, the dysphagia can manifest and/or worsen as a late-effect as well, especially as one ages and may not compensate as well for their underlying difficulty swallowing.
- Oral dysphagia may impact the ability to control liquids and move food around mouth to chew and form a swallow ready ball (bolus). Xerostomia (dry mouth) may significantly exacerbate this. It may take a long time to clear food through the mouth (aka, increased oral transit time). Sensory changes may occur in the oral cavity affecting taste, enjoyment of eating/drinking, and quality of life (Mittal et al, 2003).
- Pharyngeal dysphagia is having difficulty clearing food, liquid, and/or pills safely and efficiently through the throat and into the esophagus. It is also important to note that sensory changes or a loss of sensation can occur in the throat, which affects the action of the swallow, as good sensory input triggers good motor output. Good sensation is vital to detect food/liquid/pills getting stuck or going down the wrong way. Hutcheson, et al (2008) reported pharyngeal impairments that can be observed by the speech-language pathologist (SLP) who performs comprehensive dysphagia evaluations or instrumental examinations (e.g., videofluoroscopic swallow study/VFSS or fiberoptic endoscopic evaluation of swallowing/FEES).
Per Hutcheson (2013a), the SLP will describe problems in:
- hyolaryngeal excursion (the hyoid and larynx moving up and forward to prevent material from going down the wrong way),
- base of tongue retraction (ability of the back of the tongue to push and contact the back wall of the throat to strip the food/liquid down), and
- epiglottic retroflexion (the complex series of actions and pressures to flip the epiglottis down to help close the larynx or top of the airway),
- resulting in impairments in swallow safety (causing food/liquid to have penetration into the top of the airway/larynx and drop below the vocal cords to the lungs in an aspiration), and
- swallow efficiency (causing significant residue throughout the mouth, throat, and/or esophagus after the swallow, which can also put someone at risk for aspiration and choking/airway blockage).
-
- During the 3 to 12 months after radiation (post-radiation), the individual’s dysphagia is most likely attributable to the presence of fibrosis (stiffening of tissues and muscles) or edema (swelling). There may also be evidence of strictures, or narrowing, within the esophagus.
-
- The sequelae or negative outcomes of dysphagia may include: coughing, respiratory distress, shortness of breath, exacerbation of other respiratory ailments, lung or bronchial inflammation, aspiration pneumonia, and choking (Surkin et al, 2013).
- Esophageal-related dysphagia due to toxicity often manifests as fibrosis of the esophagus, resulting in significant limitations in overall intake. According to Chen et al (2010), at 3 months post treatment, 30% of individuals showed evidence of severe esophageal toxicity. Of the 60 individuals who underwent endoscopy, 24 showed evidence of stricture, primarily at the region of the upper esophageal sphincter or upper esophagus. Radiation esophagitis or inflammation of the esophagus is common more so in the acute phases and can be especially present in those who receive radiation for lung and breast cancer. According to Chen and colleagues (2010), risk factors for esophageal toxicity in general include:
- presence of hypopharyngeal tumors (i.e., tumors located in the lower throat close to the top of the esophagus),
- primary tumor stage of T4, and chemotherapy and radiation (aka, chemoradiation).
Summary of These Side-effects / Toxicities:
| ACUTE (<90 days post RT) | CHRONIC (>90 days to 5 years) | LATE (5 years + post RT) |
| Dermatitis | Oropharyngeal & Esophageal Dysphagia | Oropharyngeal & Esophageal Dysphagia |
| Mucositis | Osteroradionecrosis (ORN) | Cranial neuropathy |
| Pain with chewing/swallowing | Trismus | Trismus |
| Xerostomia | Xerostomia, Dental Caries | |
| Taste Changes | Taste Changes | |
| Early Signs of Dysphagia | Systemic Changes | |
| Neck/Shoulder Dysfunction | ||
| Ototoxicity | ||
| Lymphedema | ||
| Hypothyroidism |
What is Late RAD?
In the past, most research focused on the first 3 months after participants had chemoradiation therapy or only radiation therapy. However, within the last decade more focus has been on individuals who seem to develop dysphagia in the years after radiation. Some people first experience recovery of swallowing function with swallowing therapy in the 3 to 6 months after completion of their radiotherapy. Then, after 5 years, some people have found a worsening in their swallowing safety and efficiency. Researchers and clinicians have labeled this as “late-radiation-associated dysphagia,” or late RAD.
Late RAD is the development or progression of dysphagia in people who are cancer-free/disease-free but who received radiotherapy or chemoradiation for the treatment of head and neck cancer five or more years previously. The etiology for late RAD is attributable to the development of fibrosis and, in rare cases, cranial neuropathies (Hutcheson et al, 2014).
Fibrosis and Late RAD:
Fibrosis is thought to be the primary etiology for the development of late RAD. Fibrosis results in reduced range of motion and stiffness present in connective tissues and muscles of the aerodigestive tract, as well as the head, neck, and shoulders (Murphy & Gilbert, 2009; Ramia et al, 2022; Hutcheson et al, 2014). Fibrosis results from DNA damage and tissue inflammation. Once cells are damaged, they release molecules that trigger inflammatory cells such as neutrophils, monocytes, and lymphocytes. Neutrophils then trigger the release of cytokines, which directly cause fibrosis. Monocytes change to macrophages, which then stimulate fibroblasts and secrete transforming growth factor beta (TGF-β), which also further contributes to the development of fibrosis. TGF-β can cause the secretion of more collagen, which then can lead to increased rigidity and thickness, and eventually a reduction in vascularity, which further contributes to necrosis, atrophy, and loss of function. Additionally, this damage can be seen at the neuromuscular level, which has been described as “radiculo-plexo-neuro-myopathy” or “radiation fibrosis syndrome.” These radiation effects are even present in sub-structures, such as at the level of the nerves, nerve roots, and muscle fibers (Stubblefield, 2011).
Risk Factors of Late Radiation-Associated Dysphagia (Late RAD):
Increased risk factors for the development of Late RAD include (Mittal et al, 2003):
- Total radiation dose (total amount of radiation prescribed by the oncologist over the course of treatment) (Misher, 2023).
- Fraction size (total dose of radiation divided into small doses, which is given each day/each session) (Misher, 2023).
- Volume of radiation (area of radiotherapy) (Burnet et al, 2004).
- Interfraction interval (time interval between fractions given) (Marcu & Bezak, 2009).
- Type of treatment or treatment techniques (e.g., concurrent chemoradiation, types of chemotherapy drugs, and use of Intensity-Modulated Radiation Therapy / IMRT versus conventional radiotherapy techniques) (Mittal et al, 2003).
- Tissue to dose compensation (the radiation beam is altered so to provide a uniformed dose to the targeted area) (Mittal et al, 2001).
- Size or site of primary tumor.
- Engagement in smoking during and following radiotherapy (Mittal et al, 2003).
Additional factors include baseline dysphagia (as confirmed by instrumental swallow testing before radiation starts) and a total dose of 70 Gy or higher to swallowing musculature (Gharzai et al, 2020; Hutcheson et al, 2012).
Cranial Neuropathies and Late RAD:
Radiation-associated cranial neuropathies are a rare side effect of radiotherapy, resulting in damage to the cranial nerves (Hutcheson et al, 2012). The hypoglossal nerve (cranial nerve/CN XII) and the vagus nerve (CN X) are the most commonly involved cranial nerves, which can lead to vocal fold paresis or atrophy (Dong et al, 2019). Lower cranial neuropathy has been documented primarily in individuals with nasopharyngeal cancer (Hutcheson et al, 2018). The theory behind cranial neuropathy is the compressive impact of fibrosis on nerve tracts, resulting in denervation or devascularization of the nerve sheath (Hutcheson et al, 2014).
The incidence of cranial neuropathy has been estimated at 5-14% at a median follow up of 5.7-7.7 years, which generally contributes to progressive dysphagia (Hutcheson et al, 2018; Dong et al, 2019; Awan et al, 2014). Dong and colleagues (2019) reported the development of neuropathy in 14% of individuals and the median time to development of 7.7 years after chemoradiation was completed.
Factors that increase the risk of cranial neuropathy include:
- concurrent chemotherapy with increased radiation dosage and post-radiation neck dissection (Dong et al, 2019).
- dosage of radiation to the superior pharyngeal constrictors. Awan, et al (2014) reported a correlation between increased mean dosage of radiation to the superior pharyngeal constrictors (>65 Gy) and the development of cranial neuropathy.
Dysphonia is also a reported late-stage side effect. This can be impacted by the presence of cranial neuropathies, specifically, vocal fold paresis (from CN X). There are reports of dysphonia occurring up to 7 years post treatment (Patterson, 2019).
Reducing Late Radiation-Associated Dysphagia (Late RAD):
A reduction in dosage to dysphagia/aspiration-related structures (DARS) has been suggested to minimize the risk of late RAD. DARS include the palate, lingual muscles, mylohyoid, geniohyoid, genioglossus, palatoglossus, buccinator, anterior and posterior digastrics, medial and lateral pterygoid, pharyngeal constrictor muscles, supraglottis and glottis, and upper esophageal sphincter. A limitation of 60 Gy to the pharyngeal constrictors and larynx has been recommended (De Felice et al, 2018).
Dysphagia Evaluation in Radiation-Associated Dysphagia (RAD)
Severe oropharyngeal dysphagia can manifest as a result of fibrosis, atrophy, and in rare cases, cranial neuropathy.
The following are the most common impairments for the speech-language pathologist to identify and rate their severity (Mittal et al, 2003; Lazarus et al, 1993; Wu et al, 2000; Eisbruch et al, 2002):
- velopharyngeal dysfunction,
- reduced base of tongue retraction,
- incomplete or absent epiglottic retroflexion,
- reduced hyolaryngeal excursion (minimal upward and forward movement of the hyoid and larynx, which move as a unit to help close the airway and yank open the top of the esophagus),
- incomplete laryngeal vestibule closure, and
- reduced upper esophageal sphincter opening
All these deficits can result in significant safety issues (i.e., silent aspiration – where the person does not feel liquid and material going down the wrong way into the trachea and lungs) and efficiency issues (i.e., significant residue that one may not feel either). Langerman et al (2007) reported 25% of individuals who underwent chemoradiation for oropharyngeal cancer demonstrated gross aspiration as documented on a modified barium swallow study, with 75% showing silent or insensate aspiration. Thus, it is critical to fully evaluate an individual’s swallow function through an instrumental test.
Instrumental swallow evaluations are essential most of the time, especially due to poor sensation of dysphagia. The SLP will conduct a Videofluoroscopic Swallow Study (VFSS), aka, Modified Barium Swallow Study (MBSS), and/or Fiberoptic Endoscopic Evaluation of Swallowing (FEES) for a comprehensive evaluation. During testing, patterns of swallowing impairment should be analyzed (Hutcheson et al, 2018). Instrumental evaluations are recommended to fully evaluate swallow physiology, to rate the severity of the dysphagia, to determine if and why there is residue and aspiration, and to find out what can be done about it.
Please see the rating measure for Videofluoroscopic Swallow Studies titled: Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) and the DIGEST-Version 2 by Dr. Hutcheson and colleagues (2017; 2022) for rating the videofluoroscopic swallow studies by providing a safety rating, an efficiency rating, and an overall grade of severity. This is available for Flexible Endoscopic Evaluation Evaluation of Swallowing (FEES) in the DIGEST-FEES version by Heather Starmer and colleagues (2021).
In late RAD, significantly reduced or absent hyolaryngeal excursion can directly influence upper esophageal sphincter (UES) opening. People will complain of food getting stuck and potentially food and liquid falling into the airway. This is due to pooling of residue in the lower throat (within the pyriform sinuses just prior to the esophageal inlet). Many physicians may only diagnose a “stricture” or “UES dysfunction.” However, if there is minimal upward and forward movement of the hyoid bone and larynx, the top of the esophagus or UES may not be yanked open enough to allow for the ball of food (bolus) to clear into the esophagus. Therefore, just treating this with esophageal dilation alone (per a gastroenterologist’s or otolaryngologist’s endoscopic dilation) may not be effective at improving the dysphagia and symptoms (Hutcheson et al, 2012; Hutcheson et al, 2018).
Comprehensive dysphagia evaluations help target treatments by the whole medical team. It should be noted that the person is at the center of this medical team and treatment plans are discussed and created with the person’s goals of care and wishes/preferences at the center.
More Evaluation Specifics for People with Late Radiation-Associated Dysphagia:
When assessing individuals with suspected late radiation effects that may be causing dysphagia (i.e., late RAD), a thorough case history and evaluation should be conducted.
For a thorough case history, please see patient reported outcome measures (PROMs), such as the MD Anderson Dysphagia Inventory (MDADI).
A brief survey that anyone can take to screen for dysphagia and quality of life impact is the EAT-10 by Belafsky and colleagues (2008), and forms are readily available via online searches. However, there is research suggesting that the EAT-10 is not sufficient for this population with head and neck cancer (Bofill-Soler et al, 2021; Sinn et al, 2020)
Initial evaluation includes a cranial nerve examination, motor speech and voice assessments, and clinical swallow examination. Observe the lingual, labial, jaw, palate, and facial movements for symmetry, coordination and strength. Note if there are fasciculations in the tongue. In individuals who have undergone treatment for nasopharyngeal cancer, there are case studies reporting myokymia or quavering, of the tongue as a late-stage radiation effect (Poncelet et al, 1996; Tiftikcioglu et al, 2016). Areas of the submental muscles (under chin) and neck should be palpated for the assessment of fibrosis.
In addition to fibrosis, disuse atrophy can be due to a lack of swallowing activity as a result of prolonged NPO(nothing by mouth) status. Disuse atrophy may occur when one does not use the chewing and swallowing muscles (i.e., “use it or lose it” concept), and this can exacerbate or cause dysphagia (Langmore et al, 2012).
Additionally, trismus should be tested and documented. A general rule for trismus is the “three finger” test, where a person stacks three fingers between the upper and lower incisors/front teeth. An inability to maintain this opening is indicative of possible trismus. There are measurement tools and treatment devices on the market for trismus.
Fully assess the oral cavity and the person’s reports of xerostomia. See Dr. Rogus-Pulia’s research listed below in the references section regarding salivary flow and perceived dry mouth. For example, Rogus-Pulia and colleagues (2018) noted that perceived mouth dryness was associated with greater perceived swallowing effort.
Dysphagia Treatment Options for Radiation-Associated Dysphagia
Intensive swallow rehabilitation with the use of progressive resistance is cited as an emerging intervention in response to late RAD. Intensive programs aim to increase mobility of swallow musculature. Although fibrosis cannot be reversed, intensive programs may result in functional gains in overall swallow function.
Eat and Exercise is a preventative approach in which individuals undergoing cancer treatment are encouraged to maintain an oral diet (“eat”) while completing prophylactic exercises (“exercise”). Eating is paired with exercise. Outcomes suggest decreased gastronomy tube dependence, improved quality of life, and a reduced severity of dysphagia as evidenced on post treatment instrumental swallow testing (Barbon et al, 2022; Hutcheson et al, 2013b).
A swallowing specialist (SLP) can help guide a person to maintain oral intake through radiation and after in a staircase like fashion. People may need to go down the staircase at times to mainly liquids and smoothies (e.g., during and right after radiation). (See this article of tips for people with head/neck cancer that includes a recipe for smoothies.) Then the person will be guided back up to more and more regular food textures over time. See the 2020 project and article from Dr. Hutcheson and colleagues from MD Anderson collaborating with Dr. Martino and colleagues from University of Toronto regarding Eat All Through Radiation Therapy (EAT-RT). There is an image of this EAT-RT staircase within this head and neck cancer handout.
See the International Dysphagia Diet Standardisation Initiative (IDDSI.org) — for a more complete gradations in a diet framework for solids and liquids. IDDSI includes testing methods to determine: the thickness of liquids, softness of foods, particle sizes, and the food’s moistness/slipperiness. There are rationales for and descriptions of foods going from liquidised (blenderized) purees, to puree, to minced & moist, through soft & bite-sized and easy to chew foods, and finally up to all regular textures. More IDDSI Resources and Updates.
Examples of exercise programs that are eating/swallowing-driven include the following:
- McNeill Dysphagia Therapy Program (MDTP), which is focused on progressively overloading swallow muscles through swallowing (Crary et al, 2012). Emerging evidence suggests a boot camp approach to swallowing may facilitate improved outcomes.
- Intensive Dysphagia Rehabilitation (IDR) is a 2–3-week program, in which individuals attend daily sessions (Malandraki & Hutcheson, 2018). This program combines “challenge swallows” where individuals swallow a variety of difficult viscosities using a neuroplasticity approach, as well as exercises designed to strengthen specific muscle groups such as lingual or suprahyoid.
- Boot-camp: Another type of program is the MD Anderson Protocol, which utilizes a pre-boot camp or optimization phase followed by a 2–3-week bootcamp (Malandraki & Hutcheson, 2018 reviewed). During the optimization phase, individuals will receive esophageal dilation (if they are candidates), undergo Expiratory Muscle Strength Training (EMST) to target cough strength, or complete a lingual strengthening program such as the Iowa Oral Performance Instrument (IOPI) with the goal of optimizing or enhancing the outcomes of the bootcamp. During bootcamp, daily therapy sessions focus on an MDTP approach, in which individuals swallow a large number and variety of PO trials under the supervision of a Speech Language Pathologist.
Intensive exercise that is device-driven has had positive effects as well, such as: lingual strengthening and skill training with the Iowa Oral Performance Instrument, or IOPI, or the Tonguometer, or Expiratory Muscle Strength Training (EMST) to increase the strength of volitional and reflexive cough (Curtis et al, 2023; Van den Steen et al, 2020; Hutcheson et al, 2018; Hutcheson et al, 2018).
Home exercise programs may not be as effective in comparison to intensive swallowing rehabilitation with a Speech Language Pathologist, as the goal of intervention is to progressively overload swallow muscles through intensive exercise (Hutcheson et al, 2018).
Other medical interventions include esophageal dilation and vocal fold medialization, which may optimize gains seen from participation in intensive swallow rehabilitation, but ultimately will not fully resolve dysphagia.
Lymphadema treatment is completed by a certified lymphedema therapist (CLT) who is specially trained in the head and neck cancer population. During treatment, a combination of manual lymph drainage (MLD) is completed by a therapist or may be taught to the individual so that they can complete a home exercise program. Additionally, compression or wrapping is applied to the affected areas (Smith & Lewin, 2010).
Prophylactic exercises or “prehabilitation” are an emerging recommendation to prevent or reduce the severity of late RAD (Hutcheson et al, 2012). Evidence is still emerging regarding “prehabilitation” or the use of swallowing exercises prior to chemoradiation treatment with the goal of improving swallowing outcomes. Carnaby-Mann and colleagues (2012) suggested some degree of preserved function of the swallowing musculature in individuals who underwent chemoradiation and adhered to a daily exercise regimen prior to and during treatment. Hopefully, more research will provide insight into the effectiveness of prophylactic exercises in mitigating toxicities on overall swallowing function (see PRESTO study). Recent literature indicates some improvement in the short term, especially in terms of quality of life and mortality rates; however, there is a need for a larger and more robust evidence base, notably with a focus on long-term outcomes (Seth et al, 2024; Vester et al, 2023).
See more blogs about head and neck cancer dysphagia treatment:
Summary of Radiation-Associated Dysphagia: The Takeaway
With the growing number of cases of HPV-positive oropharyngeal cancer, medical Speech-Language Pathologists are tasked with management of individuals with significant impairments, not only in deglutition (swallowing), but with other debilitating side effects of chemoradiation. It is critical for Speech-Language Pathologists, as essential members of the medical team, to fully understand the etiology of, and how to identify, assess, and treat, late radiation-associated dysphagia. Additionally, when individuals with dysphagia from head and neck cancer and its treatments are aware of these issues, they and their caregivers/loved ones can advocate for themselves for early identification and early treatment. More research is underway regarding intensive therapy programs and if exercise before, during and after chemoradiation can improve swallowing for the longer term. Stay tuned and stay hopeful. Keep asking questions like our case example at the beginning of this article and advocating for yourself or your loved one with head and neck cancer – not just in April (Head and Neck Cancer Awareness Month) but all year.
*******
Links for April Head and Neck Cancer Awareness Month
- https://www.aacr.org/patients-caregivers/awareness-months/head-and-neck-cancer-awareness-month/
- https://www.headandneck.org/awareness-month/
- https://cancerblog.mayoclinic.org/2024/04/11/hpv-related-head-and-neck-cancer-treatment-is-improving-but-prevention-is-best/
More Consumer Resources
- Dysphagia Outreach Project has a Food Bank and you can apply for assistance to obtain foods, supplies and products needed: Go to https://www.dysphagiaoutreach.org
- National Foundation of Swallowing Disorders (NFOSD) provides support groups, education, and advocacy to foster “hope and improve quality of life for those suffering from all types of swallowing disorders.” Go to: https://swallowingdisorderfoundation.com
Thank you to our guest writer

Allison Hughes Bartholow, MS, MS, CCC-SLP is a speech-language pathologist at UPMC Presbyterian Hospital in Pittsburgh, Pennsylvania, working primarily with the Head and Neck Cancer population. Allison has experience working in both the rehabilitation and acute care settings, with a special interest in dysphagia and instrumental swallow examinations, including Modified Barium Swallow Studies (MBSS) (aka, Videofluoroscopic Swallow Studies) and Fiberoptic Endoscopic Evaluation of Swallowing (FEES). Contact Allison on LInkedIn here: https://www.linkedin.com/in/abartholowslp/
Authors have nothing to financially declare in sharing all links and resources.
References for Radiation-Associated Dysphagia & Head and Neck Cancer:
AAOM Writing Group. (2015, January 22). Oral mucositis. The American Academy of Oral Medicine. https://www.aaom.com/index.php?option=com_content&view=article&id=149%3Aoral-mucositis&catid=22%3Aindividual-condition-information&Itemid=120
Awan, M. J., Mohamed, A. S., Lewin, J. S., Baron, C. A., Gunn, G. B., Rosenthal, D. I., Holsinger, F. C., Schwartz, D. L., Fuller, C. D., & Hutcheson, K. A. (2014). Late radiation-associated dysphagia (late-RAD) with lower cranial neuropathy after oropharyngeal radiotherapy: a preliminary dosimetric comparison. Oral Oncol, 50(8), 746-752. https://doi.org/10.1016/j.oraloncology.2014.05.003
Barbon, C. E. A., Peterson, C. B., Moreno, A. C., Lai, S. Y., Reddy, J. P., Sahli, A., Martino, R., Johnson, F. M., Fuller, C. D., & Hutcheson, K. A. (2022). Adhering to Eat and Exercise Status During Radiotherapy for Oropharyngeal Cancer for Prevention and Mitigation of Radiotherapy-Associated Dysphagia. JAMA Otolaryngol Head Neck Surg, 148(10), 956-964. https://doi.org/10.1001/jamaoto.2022.2313
Baudelet, M., Van den Steen, L., Duprez, F., De Bodt, M., Deschuymer, S., Goeleven, A., Hutsebaut, I., Marien, S., Meersschout, S., Nevens, D., Nuyts, S., Peeters, M., Specenier, P., Van den Brekel, M., van der Molen, L., Vandenbruaene, C., Vanderveken, O., Van Dinther, J., Van Laer, C., . . . Member of the Belgian, P. G. (2020). Study protocol for a randomized controlled trial: prophylactic swallowing exercises in head-and-neck cancer patients treated with (chemo)radiotherapy (PRESTO trial). Trials, 21(1), 237. https://doi.org/10.1186/s13063-020-4171-0
Belafsky, P. C., Mouadeb, D. A., Rees, C. J., Pryor, J. C., Postma, G. N., Allen, J., & Leonard, R. J. (2008). Validity and reliability of the Eating Assessment Tool (EAT-10). The Annals of otology, rhinology, and laryngology, 117(12), 919–924. https://doi.org/10.1177/000348940811701210
Bofill-Soler, N., Guillen-Sola, A., Marco, E., Nieto-Cadalso, S., Barrera, M. C., Pera-Cegarra, O., Membrive, I., Duran, X., & Foro, P. (2021). Is EAT-10 Useful to Assess Swallowing during the Chemo-Radiotherapy Phase in Patients with Head and Neck Cancer? A Pilot Study. The Annals of otology, rhinology, and laryngology, 130(7), 689–698. https://doi.org/10.1177/0003489420966625
Burnet, N. G., Thomas, S. J., Burton, K. E., & Jefferies, S. J. (2004). Defining the tumour and target volumes for radiotherapy. Cancer Imaging, 4(2), 153-161. https://doi.org/10.1102/1470-7330.2004.0054
Carnaby-Mann, G., Crary, M. A., Schmalfuss, I., & Amdur, R. (2012). “Pharyngocise”: randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int J Radiat Oncol Biol Phys, 83(1), 210-219. https://doi.org/10.1016/j.ijrobp.2011.06.1954
Chen, A. M., Li, B. Q., Jennelle, R. L., Lau, D. H., Yang, C. C., Courquin, J., Vijayakumar, S., & Purdy, J. A. (2010). Late esophageal toxicity after radiation therapy for head and neck cancer. Head Neck, 32(2), 178-183. https://doi.org/10.1002/hed.21164
Chen, A. Y., Frankowski, R., Bishop-Leone, J., Hebert, T., Leyk, S., Lewin, J., & Goepfert, H. (2001). The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Archives of otolaryngology–head & neck surgery, 127(7), 870–876. https://pubmed.ncbi.nlm.nih.gov/11448365/
Common Terminology Criteria for Adverse Events (CTCAE) Version 5. Published: November 27. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute.
Crary, M. A., Carnaby, G. D., LaGorio, L. A., & Carvajal, P. J. (2012). Functional and physiological outcomes from an exercise-based dysphagia therapy: a pilot investigation of the McNeill Dysphagia Therapy Program. Arch Phys Med Rehabil,93(7), 1173-1178. https://doi.org/10.1016/j.apmr.2011.11.008
Curtis, J. A., Mocchetti, V., & Rameau, A. (2023). Concurrent Validity of the IOPI and Tongueometer Orofacial Strength Measurement Devices. Laryngoscope, 133(11), 3123-3131. https://doi.org/10.1002/lary.30782
De Felice, F., de Vincentiis, M., Luzzi, V., Magliulo, G., Tombolini, M., Ruoppolo, G., & Polimeni, A. (2018). Late radiation-associated dysphagia in head and neck cancer patients: evidence, research and management. Oral Oncol, 77, 125-130. https://doi.org/10.1016/j.oraloncology.2017.12.021
Dong, Y., Ridge, J. A., Ebersole, B., Li, T., Lango, M. N., Churilla, T. M., Donocoff, K., Bauman, J. R., & Galloway, T. J. (2019). Incidence and outcomes of radiation-induced late cranial neuropathy in 10-year survivors of head and neck cancer. Oral Oncol, 95, 59-64. https://doi.org/10.1016/j.oraloncology.2019.05.014
Eisbruch, A., Lyden, T., Bradford, C. R., Dawson, L. A., Haxer, M. J., Miller, A. E., Teknos, T. N., Chepeha, D. B., Hogikyan, N. D., Terrell, J. E., & Wolf, G. T. (2002). Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys, 53(1), 23-28. https://doi.org/10.1016/s0360-3016(02)02712-8
Gharzai, L. A., Li, P., Schipper, M. J., Yao, J., Mayo, C. S., Wilkie, J. R., Hawkins, P. G., Lyden, T., Blakely, A., Ibrahim, M., Schonewolf, C. A., Shah, J., Eisbruch, A., Casper, K., & Mierzwa, M. (2020). Characterization of very late dysphagia after chemoradiation for oropharyngeal squamous cell carcinoma. Oral Oncol, 111, 104853. https://doi.org/10.1016/j.oraloncology.2020.104853
Hutcheson, K. A., Barringer, D. A., Rosenthal, D. I., May, A. H., Roberts, D. B., & Lewin, J. S. (2008). Swallowing outcomes after radiotherapy for laryngeal carcinoma. Arch Otolaryngol Head Neck Surg, 134(2), 178-183. https://doi.org/10.1001/archoto.2007.33
Hutcheson, K. A., Barrow, M. P., Plowman, E. K., Lai, S. Y., Fuller, C. D., Barringer, D. A., Eapen, G., Wang, Y., Hubbard, R., Jimenez, S. K., Little, L. G., & Lewin, J. S. (2018). Expiratory muscle strength training for radiation-associated aspiration after head and neck cancer: A case series. Laryngoscope, 128(5), 1044-1051. https://doi.org/10.1002/lary.26845
Hutcheson, K. A. (2013a). Late radiation-associated dysphagia (RAD) in head and neck cancer survivors. Perspectives on Swallowing and Swallowing Disorders (Dysphagia), 22(2), 61–72. https://doi.org/10.1044/sasd22.2.61
Hutcheson, K. A., Bhayani, M. K., Beadle, B. M., Gold, K. A., Shinn, E. H., Lai, S. Y., & Lewin, J. (2013b). Eat and exercise during radiotherapy or chemoradiotherapy for pharyngeal cancers: use it or lose it. JAMA Otolaryngol Head Neck Surg,139(11), 1127-1134. https://doi.org/10.1001/jamaoto.2013.4715
Hutcheson, K. A., Lewin, J. S., Barringer, D. A., Lisec, A., Gunn, G. B., Moore, M. W., & Holsinger, F. C. (2012). Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer, 118(23), 5793-5799. https://doi.org/10.1002/cncr.27631
Hutcheson, K. A., Lewin, J. S., Holsinger, F. C., Steinhaus, G., Lisec, A., Barringer, D. A., Lin, H. Y., Villalobos, S., Garden, A. S., Papadimitrakopoulou, V., & Kies, M. S. (2014). Long-term functional and survival outcomes after induction chemotherapy and risk-based definitive therapy for locally advanced squamous cell carcinoma of the head and neck. Head Neck, 36(4), 474-480. https://doi.org/10.1002/hed.23330
Hutcheson, K. A., Barbon, C. E. A., Alvarez, C. P., & Warneke, C. L. (2022). Refining measurement of swallowing safety in the Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) criteria: Validation of DIGEST version 2. Cancer, 128(7), 1458–1466. https://doi.org/10.1002/cncr.34079
Hutcheson, K. A., Barrow, M. P., Barringer, D. A., Knott, J. K., Lin, H. Y., Weber, R. S., Fuller, C. D., Lai, S. Y., Alvarez, C. P., Raut, J., Lazarus, C. L., May, A., Patterson, J., Roe, J. W., Starmer, H. M., & Lewin, J. S. (2017). Dynamic Imaging Grade of Swallowing Toxicity (DIGEST): Scale development and validation. Cancer, 123(1), 62–70. https://doi.org/10.1002/cncr.30283
Hutcheson, K. A., Gomes, A., Rodriguez, V., Barringer, D., Khan, M., & Martino, R. (2020). Eat All Through Radiation Therapy (EAT-RT): Structured therapy model to facilitate continued oral intake through head and neck radiotherapy-User acceptance and content validation. Head & neck, 42(9), 2390–2396. https://doi.org/10.1002/hed.26250
Kearney, A. & Cavanagh, P. W. (2019). Acute and long-term effects of chemoradiation therapy in head and neck cancer. In P. C. Doyle (Ed.), Clinical care and rehabilitation in head and neck cancer. (pp. 331-349). Springer. https://www.semanticscholar.org/paper/Acute-and-Long-Term-Effects-of-Chemoradiation-in-Kearney-Cavanagh/dfd2ffbafa28f8e20afbf57900447a2a9a91a0b1?utm_source=direct_link
Langerman, A., Maccracken, E., Kasza, K., Haraf, D. J., Vokes, E. E., & Stenson, K. M. (2007). Aspiration in chemoradiated patients with head and neck cancer. Arch Otolaryngol Head Neck Surg, 133(12), 1289-1295. https://doi.org/10.1001/archotol.133.12.1289
Langmore, S., Krisciunas, G. P., Miloro, K. V., Evans, S. R., & Cheng, D. M. (2012). Does PEG use cause dysphagia in head and neck cancer patients? Dysphagia, 27(2), 251-259. https://doi.org/10.1007/s00455-011-9360-2
Lazarus, C. L. (1993). Effects of radiation therapy and voluntary maneuvers on swallow functioning in head and neck cancer patients. Clin Commun Disord, 3(4), 11-20. https://www.ncbi.nlm.nih.gov/pubmed/8111360
Malandraki, G.A., & Hutcheson, K.A. (2018). Intensive Therapies for Dysphagia: Implementation of the Intensive Dysphagia Rehabilitation and the MD Anderson Swallowing Boot Camp Approaches. Perspectives of the ASHA Special Interest Groups, 3, 133-145. https://pubs.asha.org/doi/abs/10.1044/persp3.SIG13.133
Marcu, L. G., & Bezak, E. (2009). Radiobiological modeling of interplay between accelerated repopulation and altered fractionation schedules in head and neck cancer. J Med Phys, 34(4), 206-211. https://doi.org/10.4103/0971-6203.56081
Misher, C. (2023). Fractionation and Radiation. Oncolink. https://www.oncolink.org/cancer-treatment/radiation/support/fractionation-and-radiation#:~:text=What%20are%20fractions%20of%20radiation,measured%20in%20centigray%20or%20cGy.
Mittal, B. B., Kepka, A., Mahadevan, A., Kies, M., Pelzer, H., List, M. A., Rademaker, A., & Logemann, J. (2001). Tissue/dose compensation to reduce toxicity from combined radiation and chemotherapy for advanced head and neck cancers. Int J Cancer, 96 Suppl, 61-70. https://doi.org/10.1002/ijc.10360
Mittal, B. B., Pauloski, B. R., Haraf, D. J., Pelzer, H. J., Argiris, A., Vokes, E. E., Rademaker, A., & Logemann, J. A. (2003). Swallowing dysfunction–preventative and rehabilitation strategies in patients with head-and-neck cancers treated with surgery, radiotherapy, and chemotherapy: a critical review. Int J Radiat Oncol Biol Phys, 57(5), 1219-1230. https://doi.org/10.1016/s0360-3016(03)01454-8
Murphy, B. A., & Deng, J. (2015). Advances in Supportive Care for Late Effects of Head and Neck Cancer. J Clin Oncol,33(29), 3314-3321. https://doi.org/10.1200/JCO.2015.61.3836
Murphy, B. A., & Gilbert, J. (2009). Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol, 19(1), 35-42. https://doi.org/10.1016/j.semradonc.2008.09.007
Poncelet, A. N., Auger, R. G., & Silber, M. H. (1996). Myokymic discharges of the tongue after radiation to the head and neck. Neurology, 46(1), 259-260. https://doi.org/10.1212/wnl.46.1.259
Ramia, P., Bodgi, L., Mahmoud, D., Mohammad, M. A., Youssef, B., Kopek, N., Al-Shamsi, H., Dagher, M., & Abu-Gheida, I. (2022). Radiation-Induced Fibrosis in Patients with Head and Neck Cancer: A Review of Pathogenesis and Clinical Outcomes. Clin Med Insights Oncol, 16, 11795549211036898. https://doi.org/10.1177/11795549211036898
Ranta, P., Kyto, E., Nissi, L., Kinnunen, I., Vahlberg, T., Minn, H., Haapio, E., Nelimarkka, L., & Irjala, H. (2022). Dysphagia, hypothyroidism, and osteoradionecrosis after radiation therapy for head and neck cancer. Laryngoscope Investig Otolaryngol, 7(1), 108-116. https://doi.org/10.1002/lio2.711
Rathee, M., & Jain, P. (2024). Anatomy, Head and Neck, Lateral Pterygoid Muscle. In StatPearls. https://www.ncbi.nlm.nih.gov/pubmed/31747206
Rogus-Pulia, N. M., Gangnon, R., Kind, A., Connor, N. P., & Asthana, S. (2018). A Pilot Study of Perceived Mouth Dryness, Perceived Swallowing Effort, and Saliva Substitute Effects in Healthy Adults Across the Age Range. Dysphagia, 33(2), 200-205. https://doi.org/10.1007/s00455-017-9846-7
Rogus-Pulia, N. M., Larson, C., Mittal, B. B., Pierce, M., Zecker, S., Kennelty, K., Kind, A., & Connor, N. P. (2016). Effects of Change in Tongue Pressure and Salivary Flow Rate on Swallow Efficiency Following Chemoradiation Treatment for Head and Neck Cancer. Dysphagia, 31(5), 687-696. https://doi.org/10.1007/s00455-016-9733-7
Rogus-Pulia, N. M., Pierce, M., Mittal, B. B., Zecker, S. G., & Logemann, J. (2015). Bolus effects on patient awareness of swallowing difficulty and swallow physiology after chemoradiation for head and neck cancer. Head Neck, 37(8), 1122-1129. https://doi.org/10.1002/hed.23720
Rogus-Pulia, N. M., Pierce, M. C., Mittal, B. B., Zecker, S. G., & Logemann, J. A. (2014). Changes in swallowing physiology and patient perception of swallowing function following chemoradiation for head and neck cancer. Dysphagia, 29(2), 223-233. https://doi.org/10.1007/s00455-013-9500-y
Salvo, N., Barnes, E., van Draanen, J., Stacey, E., Mitera, G., Breen, D., Giotis, A., Czarnota, G., Pang, J., & De Angelis, C. (2010). Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr Oncol, 17(4), 94-112. https://doi.org/10.3747/co.v17i4.493
Seth, I., Bulloch, G., Qin, K. R., Xie, Y., Sebastian, B., Liew, H., Rozen, W. M., & Lee, C. H. A. (2024). Pre-rehabilitation interventions for patients with head and neck cancers: A systematic review and meta-analysis. Head Neck, 46(1), 86-117. https://doi.org/10.1002/hed.27561
Sinn, F. S., Charters, E., Stone, D., Janabi, M., & Bogaardt, H. (2020). Responsiveness of the EAT-10 to Clinical Change in Head and Neck Cancer Patients with Dysphagia. International journal of speech-language pathology, 22(1), 78–85. https://doi.org/10.1080/17549507.2019.1596312
Smith, B. G., & Lewin, J. S. (2010). Lymphedema management in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg, 18(3), 153-158. https://doi.org/10.1097/MOO.0b013e32833aac21
Starmer, H. M., Arrese, L., Langmore, S., Ma, Y., Murray, J., Patterson, J., Pisegna, J., Roe, J., Tabor-Gray, L., & Hutcheson, K. (2021). Adaptation and Validation of the Dynamic Imaging Grade of Swallowing Toxicity for Flexible Endoscopic Evaluation of Swallowing: DIGEST-FEES. Journal of speech, language, and hearing research : JSLHR, 64(6), 1802–1810. https://doi.org/10.1044/2021_JSLHR-21-00014
Strojan, P., Hutcheson, K. A., Eisbruch, A., Beitler, J. J., Langendijk, J. A., Lee, A. W. M., Corry, J., Mendenhall, W. M., Smee, R., Rinaldo, A., & Ferlito, A. (2017). Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev, 59, 79-92. https://doi.org/10.1016/j.ctrv.2017.07.003
Stubblefield, M. D. (2011). Radiation fibrosis syndrome: neuromuscular and musculoskeletal complications in cancer survivors. PM R, 3(11), 1041-1054. https://doi.org/10.1016/j.pmrj.2011.08.535
Surkin, M. I., Schwartz, S. A., & Markiewicz, D. A. (2013). Late-onset complications after chemoradiation for head and neck carcinomas. Ear Nose Throat J, 92(8), E18. https://doi.org/10.1177/014556131309200822
Tiftikcioglu, B. I., Bulbul, I., Ozcelik, M. M., Piskin-Demir, G., & Zorlu, Y. (2016). Tongue myokymia presenting twelve years after radiation therapy. Clin Neurophysiol Pract, 1, 41-42. https://doi.org/10.1016/j.cnp.2016.05.002
United States Nuclear Regulatory Commission (USNRC). (2021, March). Gray (Gy). https://www.nrc.gov/reading-rm/basic-ref/glossary/gray-gy.html
Upadhya, I., Jariwala, N., & Datar, J. (2011). Ototoxic effects of irradiation. Indian J Otolaryngol Head Neck Surg, 63(2), 151-154. https://doi.org/10.1007/s12070-011-0142-9
Van den Steen, L., Baudelet, M., Tomassen, P., Bonte, K., De Bodt, M., & Van Nuffelen, G. (2020). Effect of tongue-strengthening exercises on tongue strength and swallowing-related parameters in chronic radiation-associated dysphagia. Head Neck, 42(9), 2298-2307. https://doi.org/10.1002/hed.26179
Vester, S., Muhr, A., Meier, J., Suss, C., Kummer, P., & Kunzel, J. (2023). Prehabilitation of dysphagia in the therapy of head and neck cancer- a systematic review of the literature and evidence evaluation. Front Oncol, 13, 1273430. https://doi.org/10.3389/fonc.2023.1273430
Wong, A. T. T., Lai, S. Y., Gunn, G. B., Beadle, B. M., Fuller, C. D., Barrow, M. P., Hofstede, T. M., Chambers, M. S., Sturgis, E. M., Mohamed, A. S. R., Lewin, J. S., & Hutcheson, K. A. (2017). Symptom burden and dysphagia associated with osteoradionecrosis in long-term oropharynx cancer survivors: A cohort analysis. Oral Oncol, 66, 75-80. https://doi.org/10.1016/j.oraloncology.2017.01.006
Wu, C. H., Hsiao, T. Y., Ko, J. Y., & Hsu, M. M. (2000). Dysphagia after radiotherapy: endoscopic examination of swallowing in patients with nasopharyngeal carcinoma. Ann Otol Rhinol Laryngol, 109(3), 320-325. https://doi.org/10.1177/000348940010900315
Young, D., Xiao, C. C., Murphy, B., Moore, M., Fakhry, C., & Day, T. A. (2015). Increase in head and neck cancer in younger patients due to human papillomavirus (HPV). Oral Oncol, 51(8), 727-730. https://doi.org/10.1016/j.oraloncology.2015.03.015

