 High-Flow Nasal Cannula (HFNC): Dysphagia Guidelines
High-Flow Nasal Cannula (HFNC): Dysphagia Guidelines
Does it increase dysphagia & aspiration risk?
by Karen Sheffler, MS, CCC-SLP, BCS-S of SwallowStudy.com
Introduction & Questions about High-Flow Nasal Cannula
Can I feed my patient when she is on high-flow nasal cannula (HFNC) with FiO2 of 65% and flow rate of 60 liters per minute (lpm)?
I have concerns that the high airflow will increase the risk of aspiration.
There have been many similar questions in online discussion groups, which started during the 2020 COVID-19 pandemic, but have continued years later.
NOTE: this article addresses high-flow nasal cannula (HFNC) for adults. See 14 questions in a dysphagia framework or guidelines chart at the end of this article. See also: Addendum below (before the references) for pediatric guidelines adapted from the framework guidelines presented in this article. Thank you to Sheri Rosen, MA, CCC-SLP for providing this pdf of guidelines and references for the pediatric population!
“There is no rule of thumb for this question,” responded Dr. James Coyle, PhD, CCC-SLP, BCS-S in a November, 2020 post. Coghlan & Skoretz (2017, p78) also lamented about the “paucity of the literature” regarding starting oral feeding when someone is on a high-flow nasal cannula.
That makes us uncomfortable. Speech-language pathologists (SLPs) want evidence-based answers, and we want them now. We want to do what is best for our patients in intensive care units (ICU) around the world.
Description of High-Flow Nasal Cannula (HFNC):
It is an oxygen delivery device with a wide-bore silicon nasal cannula (filling about 50% of the internal diameter of the nares per Parke, et al., 2009). It delivers high airflows of up to 60-70 liters per minute (lpm). This air is heated to body temperature (37°) and humidified (100% relative humidity / 44 mg H2O per liter) and contains a percentage of oxygen gas (i.e., FiO2 or Fraction of Inspired Oxygen) (Parke, et al., 2011). Humidification can reduce discomfort, sinus pain, and airway dryness, which can all occur with traditional oxygen delivery through a standard plastic nasal cannula.
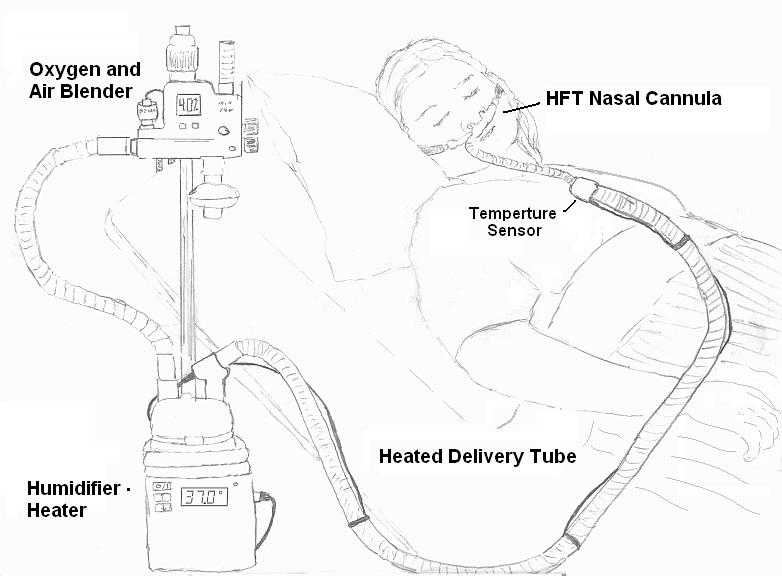
High-Flow Nasal Cannula equipment. This file is made available under the Creative Commons CC0 1.0 Universal Public Domain Dedication. https://en.wikipedia.org/wiki/File:HFT_diagram.png
So, back to this lack of definitive answers to our clinical question about high-flow nasal cannula (HFNC)…
If you have reviewed just a few articles, you may see the following quotes:
- “HFNC’s value is not limited to ventilation and oxygenation, as it may also allow for oral feeding”(Alshuwaikhat, et al., 2020). Many articles refer to the availability of the mouth, as these systems do not require a face mask.
- “Among healthy adults, regardless of age, saliva swallow frequency was not significantly affected by HFNC at any flow.” (Alshuwaikhat, et al., 2020)
- “Nasal high-flow may allow continuation of oral intake without aspiration during oxygen therapy (p 915).” That last one was really a stretch of a conclusion by Sanuki and colleagues (2017), but more on research problems later.
Or, you may start getting confused when you read:
- HFNC is common, but the “impact on the respiratory-swallowing sequence has not been closely researched, and, therefore, its impact on initiation of safe oral intake is in question…,” per Kortney Eng and colleagues (2019, p 1519).
- The “effects on swallowing are unclear,” (Flores, et al., 2019, p522).
- “HFNC may cause unwanted complications during eating and swallowing,” (Allen & Galek, 2020).
It becomes clear that we have to carefully analyze many research articles, dig through the medical record in a thorough review, and we have to know our patients well. Then, we can try to apply a set of general criteria and guidelines to each person, knowing they are an individual. Can we come up with a rough set of guidelines by the end of this article? Read on, BUT do not stop asking questions!! For example, does the population tested in a particular piece of research even apply to my specific patient?
I hope this blog will start you on your journey, and I am sorry it is so long. Our endpoint here will be to build a critical thinking framework in our SLP brains to apply to each individual who is on high-flow nasal cannula (HFNC) within a holistic person-centered care process. The framework at the end of the blog is not a researched or validated tool, and it will certainly not direct you to a diet recommendation; rather, it will guide your thinking, questions, and further testing (e.g., instrumental evaluations) so that you can demonstrate caution with these individuals who are critically ill.
What questions will you ask? I hope you will share your thoughts, experiences, and questions in the comment section below.
We are #AllinThisTogether! (check out another COVID-19 blog about teamwork required for post-extubation evaluations)
*******
Inspirations From the SLP Community for this Article on High-Flow Nasal Cannula
(no financial disclosures with these shout-outs)
Inspiration #1 – SIG13
American Speech-Language & Hearing Association’s (ASHA) Special Interest Group 13: Swallowing and Swallowing Disorders (SIG13) has an online community page where you can ask questions and contribute. There are frequent questions about starting oral intake in someone on high-flow nasal cannula. Check out these community pages at https://community.asha.org/home. For more resources and answers to your dysphagia questions, become a SIG13 member, and find the “affiliates-only online community” here: https://www.asha.org/SIG/13/.
Inspiration #2 – DysphagiaCafe
DysphagiaCafe’s Enrichment Webinar (November, 2020) titled: “Room Air to Mechanical Ventilation and Everything In-between” by Aimee Cardoso, RRT, BSRT (@Breatheeasy_RRT on Instagram). It was so informative and organized, reviewing the least to the most invasive means of respiratory support. She smartly defined key terms for us speech-language pathologists, such as FiO2, which stands for Fraction of Inspired O2. It is the amount of oxygen concentration delivered as a gas in a particular respiratory support device. Did you know that we are all on an FiO2 of 21%, meaning that room air is 21% oxygen? Cardoso noted that 100% FiO2 is not common, as alveoli damage and oxygen toxicity can happen if you are on high percentages of FiO2 (e.g., 100% FiO2 for more than 24 hours)? She reviewed airflow and the ranges of liters per minute in the common respiratory support devices; for examples:
- Traditional O2 via nasal cannula, which can only go up to 6 lpm;
- Venturi mask, which has color-coded adapters for each specific FiO2 target and goes up to 15 lpm;
- Non-rebreather/NRB, which delivers up to 90% FiO2 and has airflow rates from 10 to 15 lpm;
- High flow nasal cannula/HFNC, which can deliver up to 100% FiO2 and airflow rates of up to 60-70 lpm.
*******
Outline of this High-Flow Nasal Cannula Article
To clarify, this blog is not about how much oxygen people are receiving with their respiratory support. Instead, we want to explore the effects of the amount of airflow and how that may affect the person’s swallowing.
“It is the flow rate that is potentially problematic in terms of increasing pharyngeal airway pressure,” Coyle clarified in one recent post on SIG13 (2020).
In order to provide more information, we need to take the following outline:
A. Background on HFNC & Clarifications
-
-
- Why so many labels and acronyms?
- Why is HFNC needed?
- Potential benefits of HFNC.
- How much pressure is there?
-
B. Research on HFNC & Its Problems
1. Are study participants similar to my patient population?
2. Major problems with some prior research
a. Just because it is published…
b. Make sure to read the entire article.
C. Research on HFNC that Included Instrumental Evaluations
D. Are We Close to Guidelines?
*******
BACKGROUND ON HFNC & CLARIFICATIONS
-
WHY So Many Labels & Acronyms?
The high-flow nasal cannula (HFNC is the abbreviation I am settling on) is called by many different names, to the point that my notes on this research look like an alphabet soup! (See another blog here about the alphabet soup of pneumonias.) For examples:
- Nasal High-Flow/NHF,
- High-Flow Nasal Oxygen/HFNO,
- High Flow Oxygen Nasal Cannula/HFO2NC,
- Heated High-Flow Oxygen/HHO2.
WHY so many names? The differences in name may be due to the ability to receive high-flows via a large nasal cannula system or via an aerosol-type face mask. For purposes of this blog, we will be focusing on receiving high flows via the nose.
Many articles blindly state the one benefit of the HFNC is that the person can eat/drink just because there is no mask covering or sealed to the face, like BiPAP or CPAP (BiPAP/Biphasic Positive Airway Pressure, performing tasks of both oxygenation and CO2 clearance and CPAP/Continuous Positive Airway Pressure, which provides oxygenation).
Many people ask about the “OptiFlow™” system, and this is one brand by Fisher & Paykel Healthcare out of New Zealand that has been making high-flow nasal cannula devices since 2006 (Ward, 2013). Vapotherm™ is another brand.
We have to also clarify the alphabet soup of HFNC vs NIV vs NIPPV vs MV.
- HFNC/High-flow nasal cannula is non-invasive respiratory support. Parke and colleagues (2013) used the term of “non-invasive respiratory support” for HFNC (p 1621).
- NIV/Non-invasive ventilation versus NIPPV
- Problem: Writers sometimes refer to NIV as just BiPAP and CPAP (Singer & Rattanchaiwong, 2018), and other times they use NIV as an umbrella term for both high-flow nasal cannula and CPAP. I would advocate to separate the label of HFNC from other NIV forms for clarity, especially since there are many differences (e.g., pressure patterns are not the same). HFNC specifically has the highest pressures during the expiratory phase (Parke, et al., 2013; Sanuki, 2017).
- Cardoso (2020) recommended to use the term NIPPV (Non-Invasive Positive Pressure Ventilation) instead of NIV for specifically the BiPAP and CPAP.
- MV/Mechanical ventilation or invasive ventilation is when the patient requires endotracheal intubation to be placed on a ventilator.
-
Why are high-flow nasal cannulas needed?
Aimee Cardoso (on DysphagiaCafe, 2020, November) noted the following factors in her lecture that may indicate a person needs HFNC:
- Hypoxic (i.e., not enough oxygen in your blood to carry it to your tissues for your body to function)
- Increased work of breathing
- Increased respiratory rate
- Inadequate tidal volumes
- Need positive pressure and more support than traditional O2 therapies.
My literature review revealed the same. HFNC may be considered by the medical team when someone is in moderate hypoxemic respiratory failure that is not relieved by standard oxygen therapies (Ward, 2013; Coghlan & Skoretz, 2017). Parke and colleagues (2011) reviewed the mild to moderate hypoxemic parameters as follows:
- needing ≥ 4 L/min O2 for 4 hours, or
- ≥ 6 L/min O2 via face mask for 2 hours, and/or
- respiratory rate of ≥ 25 breaths / minute, and/or
- increased work of breathing and accessory muscle use.
Parke and colleagues (2013) described it as a good step between traditional O2 and CPAP, when needing a step-up in support or a step-down to wean off higher positive pressure devices or intubation.
-
Potential HFNC benefits
Why all this pressure and fancy equipment?
- Positive airway pressure to increase oxygenation and ventilation (Parke, et al., 2009, and Parke cited Keenan, et al., 1997, in the article on the “effects of noninvasive positive pressure”).
- Decreased nasopharyngeal and airway resistance (Gotera, et al., 2013; Parke, et al., 2009)
- Nasal airway resistance on inhalation accounts for >50% of total airway resistance (Nishimura, 2016; Coghlan & Skoretz, 2017)
- Splints airways open to decrease the work of breathing (Hernandez, et al., 2016).
- Increased resistance on expiration, which is transmitted to the lungs to work to expand lung volumes (Parke, et al., 2011; Fraser, et al., 2010).
- The higher pressure during the expiratory phase may be why nasal high flow has clinical benefits (Parke, et al., 2013, p 1623).
- Creating extrinsic PEEP, which is positive end expiratory pressure. (Gotera, et al., 2013; Cardoso, 2020; Parke, et al., 2009, stated that there is a balancing of intrinsic PEEP; Parke, et al., 2013, p 1622, noted that the PEEP is approximately 3-5 cm H2O).
- “PEEP created by nasal high flow reduces the work of breathing and improves oxygenation,” per Parke, et al., 2013, p 1623.
- Alveolar recruitment, as HFNC provides this resistance to exhalation. So when the mouth is closed, that air is transferred down to the alveoli to blow them open and prevent atelectasis (Gotera, et al., 2013; Parke, et al., 2013).
- Washes out nasopharyngeal dead space. This high airflow flushes out the expired CO2-laden air. The next inhalation can bring in more oxygen. (Gotera, et al., 2013; Coghlan & Skoretz, 2017; Roca, et al., 2016; Cardoso, 2020; Parke, 2013; Parke, et al., 2011; see also p 269 in Parke, et al., 2011 for more citations on this issue).
- Large nasal cannula and high pressure prevents diluting the FiO2 with incoming room air.
- Enhanced lung mucociliary clearance from long-term humidity and heat. Mucociliary clearance (one of my favorite words) is like the mucous and gunk in your lungs hops on an escalator of moving cilia to be swept out of the lungs (Gotera, et al., 2013; Parke, et al., 2011; Cardoso, 2020).
- Well oxygenated, even with mouth breathers (Parke, 2011).
- Reduced work of breathing (Parke, et al., 2009).
- Increased patient comfort when compared with face masks and CPAP (Coghlan & Skoretz, 2017; Parke, et al., 2011).
- Tolerated well (Parke, 2011 & Parke, 2013 – of note, these researchers disclosed that they work with the makers of the HFNC systems – Fisher & Paykel).
- Could facilitate early ICU discharge (Parke, 2011), but Parke’s 2011 research also noted that the patients’ discharge and hospital length of stay did not significantly differ.
- Fewer desaturations on HFNC versus high flow aerosol-type face mask (Parke, et al., 2011).
- Possibly improved secretion clearance, meaning from upper airways (Coghlan & Skoretz, 2017; Parke, et al., 2011). I saved this issue until the end of the list, as Dr. Jeff Ward (2013) noted that humidity could certainly help with moisture and comfort, but he had not seen any citations of published data to back up these claims in articles. I also found nothing conclusive on this topic of oropharyngeal secretion management and raising/expectorating secretions. I would also expect that the humidification could help prevent the saliva/sputum from becoming thick, sticky, and crusted on to the mucosa. Discussion of the oral cavity and eating/swallowing safety tend to be left out of the articles’ lists of benefits. We need to keep raising dysphagia awareness and keep swallowing issues in the forefront of researchers minds in these related fields. More transdisciplinary/cross-disciplinary communication will help.
-
Just how much pressure are we talking about?

Good question!
There is a positive relationship between amount of flow and amount of additional nasopharyngeal airway pressure above the standard atmospheric pressure already present.
I will start with the Parke and colleagues’ studies of 2009 and 2013. In 2009, they studied the pressures on 15 cardiac intensive care patients with pressure monitors behind and just below the uvula during an airflow of 35 lpm. They allowed for breathing patterns to “settle down” before taking measurements with a high-flow face mask versus nasal cannula, and while in the mouth open versus mouth closed positions. Highest pressures were noted with HFNC and mouth closed (2.7 cm H2O with range from 1.5 – 5.3), and the differences reached statistical significance. They noted how there was significant variability in nares sizes, causing leaking in some people, leading to less resistance to expiration and lower pressures. Parke, et al. (2013) noted that their wide ranges and lower pressures found in 2009 may have been due to taking pressure measurements across the inspiration and expiration phases. Parke and colleagues found in their 2013 study that higher pressures were in the expiration phase of the cycle, with average at 50 lpm up to 3.1 cm H2O. Their peak expiratory pressures were also flow dependent and as follows:
30 lpm 3.01 cm H2O (± 1.18)
40 lpm 3.81 cm H2O (± 1.45)
50 lpm 4.86 cm H2O (± 1.79)
(Keep in mind that standard CPAP pressures are between 4 – 20 cm H2O)
When Dr. James Coyle, PhD, CCC-SLP, BCS-S discussed this issue at the Dysphagia Research Society’s annual meeting in 2017 (session with Dr. Martin Brodsky titled: Evaluation and Treatment of Dysphagia in the ICU – see this blog that reviewed sessions from the annual meeting), he warned that some nasopharyngeal pressures reached CPAP levels. He was citing the work by Groves and colleagues from 2007, which was reiterated in Ward (2013). They found mean nasopharyngeal pressures with the mouth closed at:
30 lpm 3.7 cm H2O
40 lpm 7.2 cm H2O
50 lpm 8.7 cm H2O
(Again, this reaches pressures higher than those in the CPAP range!)
Coghlan and Skoretz (2017) also noted that pressures on expiration are highest, but they stated that overall measurements of pressures in the oropharynx are difficult to determine. They speculated that there is at least an increase of 0.5 to 1 cm H2O for every 10 lpm increase in flow.
*******
RESEARCH ON HIGH-FLOW NASAL CANNULA & ITS PROBLEMS
There are problems with aspects of HFNC research, and I think this thorough review will help you critically analyze the research findings to develop your framework and guidelines.
-
Are the study’s participants similar to the person you are evaluating?
A lot of the research may contain a population that is not representative of your patient population. For example, the often-cited Sanuki, et al., 2017 study examined the “swallowing reflex” in only 9 healthy males (Japanese). No age range was given. The Parke, et al. (2013) studied 14 males and only 1 female, and these were patients who had come in for elective cardiac surgeries in New Zealand. Additionally, that study did not address swallowing at all. The pressures analyzed in Parke, et al., 2013, were in participants without significant respiratory compromise. Their mean age was 59.5 (±10.6). Their average body mass index (BMI) was 29 ±5 (in the overweight category). What about our female patient who is frail, cachectic, sarcopenic, over 80, breathing shallow and fast, and very weak? Parke and colleagues (2013) noted how “female patients experience significantly higher airway pressures than males with OptiFlow™” testing per the Groves & Tobin (2007) study (p 1624). Even the Parke, et al., 2011 study, which at least tested more people (N=60), had all cardiothoracic and vascular ICU participants, rather than the primarily respiratory compromised patients that the SLP may typically see. That 2011 study randomized people to an OptiFlow™ HFNC group at 35 lpm versus a high-flow face mask group. The HFNC group had 90% success rate versus the 56% success rate in the face mask group. Only 3 (10%) in the HFNC group had to change to NIPPV versus 30% in the face mask group. None of the HFNC participants ever changed to the face mask method. They noted that their study was not powered enough to detect a treatment effect (p 269). Would the same success rate occur with your typical ICU patient?
If your typical ICU patient who needs a swallowing evaluation to initiate oral intake has significant respiratory compromise and significant past medical history of respiratory disease, what do you do? Eng and colleagues (2019) did a nice review of why the SLP is concerned about swallowing safety in a person with respiratory compromise. This can be summarized as follows:
- Decreased breathing and swallowing coordination. As Dr. James Coyle (2010) noted, us humans can only do one thing at a time, and the “act of swallowing is like a railroad switch,” (p 91). Will you have time between breaths to flip the switch to swallow? Will you gasp for air after the swallow and suck in a breath, along with food/liquid material? See also references below from Martin-Harris and McFarland.
- Decreased sensation of residue and aspiration.
- Decreased respiratory drive, causing an inability to forcefully eject any foreign matter. This blog cannot possibly cover all the cough research and even the blunted urge to cough seen in some patients (e.g., people with Parkinson’s disease).
It is easy to see how a person who is very compromised could have significant difficulty compensating for the high pressures present with HFNC, whereas the healthy younger participants can make adaptations to any novel and potentially disruptive stimuli (aka, adapting to a perturbation – read more about this in section #7 of this blog, where I discuss Dr. Humbert’s lecture on the cerebellum, perturbation and adaptation).
-
High-Flow Nasal Cannula & 3 Poorly Designed Studies
a. First Point: Just because it is published, doesn’t mean that it is true.
Many prior research summaries on this topic (Eng, et al., 2019 included) state that research is limited, but still report findings of poor research as if they are written in stone and without analysis. If the whole foundation of the research is faulty, can we look to it for answers? Can we risk making overgeneralizations?
In this section, I will review the research of Sanuki, et al., (2017); Oomagari, et al., 2015; and Alshuwaikhat, et al, 2020. For ease of discussion, I will refer to them by their first authors’ names. It is also so crucial to note: the Oomagari and Alshuwaikhat research is only available in an Abstract form, per my and my hospital librarian’s searching.
1) Sanuki and colleauges research (2017)
The Sanuki and colleauges research (2017) has been mentioned above, but now for the deeper dive. Don’t worry, there are some good things in this research too. They tested 9 healthy Japanese males at rest and at randomly ordered airflows of: 0 (control), 15, 30, and 45 lpm on a HFNC system. They wanted to see if there would be an effect on the latency in the swallowing reflex at the different HFNC flow rates. They placed 2 surface electrodes in the submental region (under chin) for electromyography (EMG) to tract the swallow. They recorded inspiration-expiration cycles with respiratory inductance plethysmography (RIP). Then, a catheter (8 French) was fixed to the retromolar gingiva through which they administered water. They tested boluses of 5ml over 3 seconds and a continuous “infusion” of 2ml/min. Such tiny liquid amounts! The latency time was measured from the time between the start of bolus injection to the “swallowing response” (defined as the onset of the EMG burst, along with visual inspection, which we know that looking at the neck is not so accurate). Techniques aside, their findings about respiratory-swallowing coordination are consistent with prior respiratory-swallow coordination research (see Drs. Martin-Harris and McFarland’s work in references below). They showed how the 127 studied swallows occurred across the 4 phases of the respiratory cycle on the RIP curve (see their Figure 2 & Table 3 on pages 916 and 918). Data on their control swallows was as follows:
- E swallow – swallows occurring during the expiratory phase (87.1% of the total number of control swallows [N=31] with airflow at 0 – were with this known-to-be safe pattern. Research has shown that we usually exhale-swallow-exhale as an automatic airway protection technique – see Figure 2 and Table 3 in the paper for more).
- I-E Swallow – this is swallowing in the transition between inhalation to exhalation, swallowing right before the exhale (9.7% of control swallows were this pattern).
- I Swallow – swallows during the inspiratory phase (this is dangerous, as that further inhale after the swallow could suck in any liquid or material near the airway. Only 3.2% of control swallows were this pattern).
- E-I Swallow – this is swallowing in the transition time between the exhalation and the next inhalation, swallowing right before a full inhale (this is also dangerous, and no [0%] control swallows were in this pattern – thanks to our built-in defense mechanisms).
However, the researchers never commented on these interesting patterns or discussed the potential dangers of inhaling after the swallow. I will summarize their data here in a new way, as this was not written in their text.
- 96.8% (#1 and #2 above) of the “control” swallows by healthy participants with no HFNC pressures to affect their respiratory-swallowing patterning were in this safer mode of exhaling after the swallow.
- Only 3.2% of the “control” swallows were in the potentially unsafe patterns of inhaling after the swallow (#3 and #4 above). That was with no perturbation of airflow pressures.
- Now, check out these numbers when you look at the potentially dangerous patterns of I Swallow and E-I Swallow together (inhaling after swallow) across the HFNC airflow amounts, keeping in mind those high pressures discussed above:
-
- 15 lpm: 11% of swallows potentially unsafe
- 30 lpm: 18.7% of swallows potentially unsafe
- 45 lpm: 25% of swallows potentially unsafe, inhaling after the swallow in the presence of pressures that are in the CPAP range.
-
However, Sanuki and colleauges wrote: “the timing of swallows in relation to phase of respiration (p = 0.409) were very similar under all conditions (Table 3) (p 918).” In their discussion, they reiterated that swallowing timing was “not affected” by the level of airflow with HFNC (p 918). They seemed to ignore their own data. Going from 3.2 to 25% of swallows having a potentially unsafe pattern seems at least clinically significant to me! Was this statement due to underpowered research with just 9 participants? I am no statistical wiz, so I need help here.
I haven’t even gotten to their main research question about swallowing latency! They noted that the swallows were significantly faster (i.e., smaller latency) in those with the airflow from the HFNC versus control condition (0 lpm).
0 lpm 11.9 seconds (± 3.7s)
15 lpm 9.8 seconds (± 2.9s)
30 lpm 9.0 seconds (± 2.7s)
45 lpm 8.5 seconds (± 3.9s)
The average respiratory rates were also lower, reaching significance at 30 lpm (8 breaths per minute) versus control (15). Those at 15 lpm were at 9.8 breaths per minute, and those at 45 lpm were at 10 breaths per minute. They also stated that the frequency of swallowing did not significantly improve but gave no data.
Sanuki and colleagues summarized that HFNC “may progressively enhance swallowing function with increasing levels of NHF [nasal high flow]” (p 918). However, I would suggest that it was the novelty of the high airflows and differing pressures that may have been the stimulant to enhance the speed of swallowing onset. The participants were exposed to the airflow rates in random order with an equilibrium time of only 2 minutes for each, and then the boluses were infused into this same region (posterior oropharynx). This perturbation to the system of airflow plus liquid may have ramped up their sensory input to trigger the faster motor output. The authors even noted that HFNC has fluctuating positive pressure, with pressures highest at expiration and minimal with inspiration (unlike CPAP, which is continuous positive pressure). Therefore, the authors speculated that this fluctuation may activate afferent receptors in upper airway to stimulate the swallow. Exactly. This is novel brief stimulation. The main take-home point here is that these are healthy participants who receive a novel and intrusive stimuli to alert the system, and then they adapt to this perturbation. However, what about your patients who are compromised, and the area actually becomes desensitized from long-term use of the HFNC?
My largest issue with Sanuki’s research is the over-stating of findings, as I noted in the beginning of this blog. When they link this small finding of shorter swallow latencies at higher flow rates to “enabling oral intake without aspiration,” that is a gross overstatement. They did not test for aspiration in any way. At the same time, their study Limitations section is quite small, indicating only that it was healthy adults (still not mentioning ages) and that they used distilled water, so results do not apply to food.
The reason why I have spent so many paragraphs discussing this Sanuki, et al., 2017 study, is that their findings suggest safety of oral intake with HFNC. These findings are taken as FACT and only briefly repeated rather than analyzed over the last 3 years (i.e., Flores, et al., 2019; Eng, et al., 2019; Allen & Galek, 2020). This is a dangerous issue with research.
***Thank you for reading on!
It is these details that will help you have in depth discussions with the medical team when that next consult comes in.***
2) Oomagari and colleagues (2015)
Oomagari and colleagues (2015) have research published in abstract form only, which limits our understanding. They studied 32 healthy subjects (no gender or age data provided) while under airflow rates of 0, 10, 20, 30, 40, 50 lpm. They used only the Water Swallow Test (WST) and the Repetitive Saliva Swallowing Test (RSST) as measurement tools. (Please see this blog about Dr. Leder’s research, including an actual standardized valid and reliable swallow screen that they could have used – The Yale Swallow Protocol). They indicated that 5 people “choked” at flow rates of 40 and 50 lpm. There was no definition of “choked,” but I doubt that they really had airway obstruction while swallowing water. We cannot even identify definite airway invasion at the bedside, and there was no imaging of the swallow in this study. Did they respond with a cough? Well, a cough is a good thing, and maybe they independently coughed out the liquid that poked into the top of their airway, which could be considered normal on the Penetration Aspiration Scale (PAS scores of 2 and 4).
They indicated that there was a significantly lower number of swallows at 20 lpm versus 0 lpm. Therefore, participants swallowed saliva less often at the higher flow rate of 20 lpm. They noted there was also a “greater difficulty swallowing” at 20 lpm, but they never indicated that they gave the participants a rating scale to rate their effort.
Then, to add to the confusion, they noted: “change in the swallowing time was significantly associated with the difficulty swallowing at 40 and 50 lpm.” This “change in swallowing time” was found to be a predictor of “choking when HFNC is used, with an odd’s ratio (OR) of 1.02. We do not know what this “change in the swallowing time” means. They did not look at timing or latency as Sanuki did. Were these changes in the number of times participants swallowed over 30 seconds? The only hint is under methods: “The swallowing time and number of swallows in 30 seconds were evaluated during the RSST.”
Their take-home message in the conclusion over-stated their findings. They made a blanket statement that “>40 lpm was associated with decreased swallowing function in healthy subjects.”
I know! I am not helping yet
to find definitive answers…
3) Alshuwaikhat and colleagues (2020)
Alshuwaikhat and colleagues (2020) published their research in abstract form only. They stated that HFNC is an alternative to NIV, which shows how some researchers do not put HFNC in the same category as all noninvasive ventilation (as I noted above). They studied 18 healthy adults (21-83), noting that there were only 3 older subjects (55, 65, 83 years old). Then, they reported on the younger subjects only, as they indicated the older adults had the “same pattern.” They used the Spontaneous Swallowing Frequency (SSF) as a measure of how often participants would swallow their own saliva while receiving 30, 45, and 60 lpm randomly for 10 minutes each with a 10-minute break in between. Baseline rates were taken first. A KayPentax Digital Swallowing Workstation recorded “all sEMG activity” to capture the swallow, along with visual confirmation. Here are the results of how many times they swallowed per minute (SPM) as reported:
0 lpm 1.12 SPM (SD=0.75)
30 lpm 1.13 SPM (SD=0.79)
45 lpm 3.91 SPM (SD=10.26)
60 lpm 1.01 SPM (SD=0.69)
They only commented that no statistical significance was found, but the study was underpowered, and data presented are only on the 15 younger subjects. Why would they not comment on the high variability that occurred at 45 lpm? Why does the swallowing frequency go down at 60 lpm? Why is the baseline rate higher than averages per two studies by Crary and colleagues in 2013 (Spontaneous Swallowing Frequency 2013a norms: younger: 0.47 vs. older: 1.02 swallows per minute; 2013b norms: mean age 56 – 0.56 SPM)? Did they take the baseline/no flow measurements with the device already in place, acting as sensory stimulation already?
They conclude that the spontaneous swallowing frequency was NOT affected by HFNC “at any flow.” Really?
These 3 poorly designed studies
have left us at potentially:
2 Pro-HFNC + eating without concerns,
and 1 Con-HFNC + eating.
And they could all be thrown out.
b. Second Point: Make sure to read the whole original article
(when it is available, unlike Alshuwaikhat and Oomagari above)
This is crucial in the case of Leder, et al., 2016.
Some editorials that are in favor of simply starting oral intake on people with HFNC are based on errors from not carefully reading the original article’s methods. For example, Singer & Rattanachaiwong (2018) ask in their title: “To eat or to breathe? The Answer is Both!” They answered this question by erroneously describing Leder, et al., 2016. Unfortunately, they wrote: “using high-flow nasal oxygen (HFNO) administration allowed complete oral alimentation in ALL the patients included in a recent study” (p 2 of 3). Fortunately, the review by Coghlan & Skoretz (2017) reviewed Leder’s research more closely to see that 1 in 5 adults on HFNC were NOT appropriate for oral intake (only 39 out of 50 adults [22%] were stable enough to move on to oral trials.
Let’s look at this work by Leder and colleagues (2016) more closely, as I remember discussing it in the ICU when this article came out. I frequently noted that this was the only piece of literature we had to back up our practice in people on HFNC. They studied swallowing in 50 neonates and 50 adults on HFNC. I will address the adult portion only. The details of the study design are the key (and this is similar to Dr. Leder’s exclusionary criteria in his Yale Swallow Protocol research, which I personally discussed and confirmed with him at several Dysphagia Research Society meetings prior to his passing in 2016). We have to know about who is excluded and why, so that the clinician knows to use similar exclusions in the ICU. It is all in the decision-making criteria listed on page 156 of Leder, et al., 2016. Only 39 of the 50 participants were “deemed medically appropriate to resume oral feedings.” 11/50 were NOT appropriate due to severe respiratory or cognitive issues. Those are the details we need to know about.
The article notes that the following medically appropriate criteria were met per the intensivist, the SLP and nursing for those 39 out of 50 adults:
- Stable respiratory status on 10-50 lpm of HFNC,
- Adequate mental status to participate at mealtimes,
- Passing the Yale Swallow Protocol/swallow screen (which includes the cognition testing of oriented and following commands, as well as drinking 3 ounces of water with consecutive sip/swallows – without stopping),
- If protocol this validated and reliable swallow screen was failed, then a FEES (Fiberoptic Endoscopic Evaluation of Swallowing) was performed (a comprehensive instrumental swallowing evaluation).
- Ability to manage their own secretions.
Extensive discussions, a validated swallow screen, and even full instrumental evaluations as needed were what was necessary to place those 39 out of 50 people on a diet. Out of that 39, the 5 who failed the Yale Swallow Protocol received an immediate FEES. This endoscopic evaluation guided these patients to a modified diet and thickened liquids with safer swallowing strategies. So, this was not a slam-dunk-100% tolerance as alluded to by Singer and Rattanchaiwong.
Additionally, success of oral intake was measured only at the bedside with monitoring for overt signs or symptoms of dysphagia (e.g., cough or respiratory issues). Even if the person was eating minimal amounts, it was still counted as successful. Unfortunately, though, Leder and colleagues wrote in the article that the “Oral Feeding Success” for adults was of 100% (see Table 4 on p 158). This is the dangerous over-stating of findings in an easy-to-read chart may have been what lead Singer and Rattanachaiwong (2018) to say ALL people on HFNC can be successful at eating!
Take-home questions: Who is ready? Who is not ready?
That list above is how we could narrow down our patients to determine who is appropriate for trials of oral intake. Ask who is ready and who is not ready.
With all these issues above, Leder and colleagues’ take-home message was that it is the underlying medical condition of the patient that dictates where he/she/they eat or not. Authors indicate that patients may be able to eat regardless of the fact that they require HFNC. They argue that oral intake is more dependent on the following issues than the presence of the HFNC:
- Medical condition
- Respiratory condition
- Mental status
- Physical strength and condition
(What about the specific pathophysiology of the swallowing seen on an instrumental swallowing evaluation in the presence of high airflows and pressures? Fortunately, that was looked at by Allen & Galek, 2020, but we will get to that in the next section! Read on…)
*******
HFNC RESEARCH THAT INCLUDES INSTRUMENTAL SWALLOWING EVALUATIONS
1. Flores and colleagues (2019, June)
Flores and colleagues (2019, June) performed a retrospective chart review of consecutive patients who were on high-flow nasal cannula (30-50 lpm, FiO2 35-99%) during their videofluoroscopic swallow study / VFSS (aka, modified barium swallow study / MBSS) during hospitalizations from 2015 to 2018. They studied 10 patients (44 – 92 years old, with 7 people over 65). Nine out of the 10 had no prior history of prolonged intubation or tracheotomy, with only one patient having a 5-day intubation, 5 years prior. One study patient had a brief intubation during the acute stay for <24 hours. The majority (80%) had significant prior pulmonary disease, 50% had cardiac disease, and the majority were male (70%). So these participants at least appear more similar to the SLP’s typical caseload.
However, it is so important to look at even more details of the study group. They were quite a functional group. Only 3 out of 10 were non-ambulatory due to their unstable respiratory status or weakness. Only 4 out of 10 were described as cognitively impaired with inability to follow directions or retain new information. The group must have been deemed stable enough to go to radiology for the VFSS (likely passing the criteria by Leder, et al., 2016).
Prior to their VFSS, 9 out of 10 of these patients were held NPO. Interestingly, the one person who was placed on a regular and thin liquid diet by the medical team prior to the VFSS was changed to Dysphagia Advanced (old National Dysphagia Diet, which is now Soft & Bite-Sized per IDDSI.org) and nectar thick liquid (mildly thick liquid). This reinforces the need for full discussions and swallowing evaluations before the patient is placed on a diet.
Findings: Five out of 10 (50%) had silent penetration (PAS 5) or aspiration (PAS 8). The researchers stated that 8 out of 10 patients were placed on a dysphagia diet; however, they did not use standardized IDDSI.org Framework labels and descriptions. In looking at Table 1 on page 524, it appears that ALL 10 out of 10 patients required modifications. The researchers erroneously labeled the following 2 patients’ diets as normal/regular: “Dental soft” and “Dental soft, chopped.” Those are significant modifications. This is why we have IDDSI, so that clinicians and researchers all start speaking the same standardized language for improved patient safety. Interestingly, those 2 patients on the highest diet levels had lower flow rates of only 35 and 40 lpm (not 50 lpm). They were also cognitively intact, age 44 and 73, and highly ambulatory (70-350 feet). They were feeding themselves or required only supervision (but no assistance in feeding). Whereas all 4 individuals with cognitive impairment and minimal ambulation were on liquid modifications (i.e., 3 on nectar thick/mildly thick liquid and 1 on NO liquids and just on Puree – who also was on 50 lpm & 99% FiO2. That 1 patient was also non-ambulatory due to unstable respiratory status). A total of 7 out of 10 patients were on thickened liquids.
A total of 6 out of the 10 went on to have success with their diet modifications. The one person that they tried a Free Water Protocol with to give thin liquids between meals (meals being dysphagia advanced/Soft & Bite-Sized and nectar thick/mildly thick liquid) was the one who declined due to an aspiration pneumonia. Another patient did not meet criteria for PO tolerance due to worsening respiratory distress, but a direct connection to aspiration pneumonia could not be determined. The final 2 out of 10 were changed to comfort measures only and the diet was liberalized.
Take-home messages: In a fairly high-functioning group, still 50% had silent penetration/aspiration. Flores and colleagues speculated that this constant positive airway pressure from high-flow nasal cannula may lead to a “desensization effect” at the level of the peripheral and/or central nervous system response and due to this constant sensory stimulation (p 526). Additionally, people need to have an adequate expiratory force to expel any matter that enters the top of the airway (larynx and trachea). However, the high expiratory pressure from HFNC may prevent such expectoration. Likely due to both of these issues, 70% of patients required thickened liquids. So, yes to oral intake, but with significant caution and modifications.
2. Eng, Flores, and other colleagues (2019, December)
Eng, Flores, and other colleagues (2019, December) went on to study HFNC further. They examined 80 healthy subjects (31 men and 49 women). Their ages ranged from 35-65 years old. After a few people dropped out, there were 21 people in the 35-44 age group; 27 people in the 45-55 age group; and 30 in the 55-65 age group. They tested participants with the VFSS/MBSS while on airflows of 0, 20, 40, 60 lpm on Optiflow™ with the nasal cannula occluding 50% of the nares. Note: These healthy participant studies tend to use an FiO2 of 21% (which, remember, is the same as room air). They hypothesized that a more impaired MBSImP performance would occur as the flow rate increased. They found that the flow rate affected only:
-
- Total MBSImP score at 60 lpm
- Lip Closure. This makes sense, as the pressures are greatest with the mouth closed as noted above in this blog. They found that these healthy participants were compensating by keeping their mouth open to relieve the pharyngeal pressures. This may have made all the oral scores worse (tongue control and oral residue as well).
- Tongue Control
- Oral Residue scores
Take-home messages: The researchers noted that the MBSImP scores changed orally but not for the pharyngeal components. They speculated that healthy subjects, who are cognitively intact with no co-morbidities, can adapt and compensate in the presence of these high pressures. Because they were healthy and <65 years of age, they don’t really change significantly from their baseline in the presence of high pressure.
It seems to be all about WHO CAN ADAPT?
3. Allen & Galek (2020)
Allen & Galek (2020) thoroughly analyzed the videofluoroscopic swallow study results of 29 healthy participants who were on high-flow nasal cannula airflow settings of 10, 20, 30, 40, 50, and 60 lpm in randomized and blinded order presentations. These “younger adults” were 23 females and 6 males, all younger than 60. They used 40% weight/volume Thin Liquid Varibar Barium Sulfate Suspension™ (Bracco Imaging), rather than a 20% ultra-thin solution. The standard 40% is a little bit thicker in viscosity than water per Dr. Catriona Steele (personal communications). They used a 20 ml sip size, which is a good sip size to use to challenge the swallowing physiology. Baseline measures were taken at 0 lpm to represent control swallows. Participants held the bolus in the mouth until given the command to swallow the 20 ml with one swallow. That type of command could affect the normal timing of the swallow and become a safer swallowing technique, but this method is typically done in research to reduce radiation time and to not miss recording the swallow. VFSSs done in the clinic should use more independent and natural drinking timing without commands.
Their outcome measures were Penetration-Aspiration Scale (PAS) and the duration of laryngeal vestibule closure (dLVC). I highly recommend reading their thorough description of the complex sequence that is laryngeal vestibule closure. It spans across their first two pages of this article! These highly sequenced and coordinated movements typically happen within 310 to 1070 milliseconds, per the seminal research by Molfenter and Steele (2012). I want to reiterate that they are testing the duration of laryngeal vestibule closure/dLVC, looking at how long people had the top of the airway closed. They did not look at the delay to reach maximum closure (i.e., from initial hyoid burst at the start of the pharyngeal swallow response to reaching maximum closure), which could also be a major factor in the safety of the swallow and aspiration.
Because the participants were all young and healthy, all 812 swallows had complete vestibule closure, regardless of airflow rate. There were no swallows that had incomplete or absent closure.
They did not find any significant change across airflow rates in the PAS, with no PAS scores of 5 through 8. The majority were rated as PAS of 1 or 2, which are completely normal, with a 1 meaning no penetration and a 2 meaning penetrated material pokes into the top of the laryngeal vestibule but is spontaneously ejected. A PAS of 3 (penetration above the level of the vocal cords, and not ejected) occurred 8 times, but without a notable pattern (i.e., 2x at 10 lpm, as well as 2x at 60 lpm). A PAS of 4 (meaning that the liquid penetrated to the level of the vocal cords but was ejected independently) only occurred 1x at 10 lpm and 1x at 20 lpm. Unfortunately, though, the interrater reliability for the PAS was only 22%.
Fortunately, the intra- and interrrater reliability was good for the duration of laryngeal vestibule closure. They found that when the airflow increased, the duration of laryngeal vestibule closure increased. This is an important finding and consistent with what we know about bolus volume. As the bolus volume increases, many physiological parameters of the swallow (like dLVC) increase. It reinforces the notion that WE ADAPT, especially when healthy and cognitively alert to changes in sensory input and bolus parameters. Even though these participants were blinded to the exact airflow rates, they could feel the difference. They described an increased subjective difficulty in swallowing at the higher airflow rates. That reinforces the confusing description of results in that Oomagari, et al., 2015 study that showed increased difficulty above 40 lpm. Allen and Galek also noted that the flow rates of 50 and 60 lpm showed an increased variability in timings. That variability may tip the scales for some more frail individuals to cause safety and efficiency issues with their swallows.
Take-home messages: We need to look at the specifics of the individual’s swallowing physiology in the presence of high airflow pressures. The PAS does not paint the picture. What is happening to the sensory information and the swallows’ timing, coordination, movement? In this study, there was:
- higher variability at the higher pressures,
- people reported increased difficulty swallowing, and the
- duration of laryngeal vestibule closure increased at the higher pressures.
They noted: for every 1 unit of increased airflow, the duration of closure increases by 0.002 seconds. Yes, that is significant when we are talking about durations of 310 milliseconds. These healthy younger adults are compensating and adapting to these high pressures that are trying to mess with their swallow. They are keeping their larynx closed just a little bit longer for protection. How cool is that? We adapt. Will your patient be able to adapt? If the individual in front of you in the ICU is already struggling to coordinate fast breathing (>25 breaths per minute; Cvejic, et al., 2011) with the apneic period of swallowing, will they complete the swallow safely? To help you understand this further, read this excerpt from Dr. James Coyle (2010):
“Respiratory rate deserves specific attention during evaluation. An elevated respiratory rate of 30 or greater is one of the risk factors that raises mortality risk in patients with pneumonia (Lutfiyya, Henley, Chang, & Reyburn, 2006) and can interfere with respiratory-swallow coordination. Breathing 30 times per minute means that the duration of each breath is roughly the same duration as one swallow. Each swallow, then, prevents one breath, promoting accumulation of CO2 and raising the respiratory rate even more, exacerbating the incoordination discussed above. When breathing 40 times per minute, one swallow takes longer than one breath. A patient breathing 60% oxygen (three times as much oxygen as in room air) through a facemask (using the mouth and nose) at a rate of 32 breaths per minute to maintain 89-90% saturation is working very hard to survive and may, for the moment, not be ready for eating and drinking. The patient’s underlying condition will predict the natural history of his/her recovery.” (Coyle, 2010, p 95)
*******
ARE WE READY FOR A FRAMEWORK OR GUIDELINES?

Performing good evidence-based practice requires a lot of critical appraisal of the research, as well as a thorough medical record review to really know the individual you are evaluating in the ICU. With the fast pace of healthcare, many providers may feel they do not have the time to be as thorough as is necessary.
What has not helped, though, is sharing too brief editorials, such as Singer & Rattanachaiwong (2018). These authors spent half the editorial reviewing how people on noninvasive ventilation may not be ready for oral intake because they are very sick, unstable, in respiratory distress/failure, and the team is just trying to prevent the use of invasive intubation for ventilation. However, then they made the distinction between NIV (CPAP & BiPAP) versus HFNC where the mouth is available. After incorrectly summarizing Leder and colleagues’ (2016) supposed 100% success rate, they made it seem like you could easily start to eat/drink while on HFNC. To make matters worse, they started the article claiming that holding off on oral intake is “starving” the patients. Singer & Rattanachaiwong referred to a French study that showed “nearly 60% of patients were starved during the first 2 days of treatment” (p 1). (The French study cited was Terzi, et al., 2017.)
We learned our lesson, right? One needs to read the original work by Terzi and colleagues, who analyzed 1075 people who were on noninvasive ventilation (NIV) for more than 2 days during 2000 and 2015. Findings: 57.8% had no nutritional support, 32.7% had oral intake, 2.6% had enteral nutrition (with or without additional parenteral nutrition), and 6.9% had parenteral nutrition solely. They found that fasting for 48 hours after the start of noninvasive ventilation was NOT associated with a 28-day mortality (p 7). The mortality rate, and even the amount of people who ended up on a ventilator, was higher in the enteral feeding group versus the no nutrition group.
The term “starving” should never be used by doctors when having conversations about patient safety and the risks/benefits of any nutritional decision. That is certainly not the way to phrase your discussion with the healthcare proxy (when the patient is incapacitated). Would this cause guilt to win out over reason?
What guidelines should we use instead of pressure from the team?
Many of these researchers come together around the Leder and colleagues’ concept that the presence of HFNC alone does not dictate an NPO status. Rather, it is all the factors when considered together in a holistic approach to person-centered care that guides our oral intake decision-making (Leder, et al., 2016; Eng, et al., 2019; Flores, et al., 2019; Coghlan & Skoretz, 2017).
The following chart is my summary from my literature review, as well as bringing in other related research (e.g., research behind the Yale Swallow Protocol, including the exclusionary criteria those researchers used). This chart is what we could consider in our mental framework or guidelines. The use of these guidelines requires:
- thorough chart reviews,
- discussions with the nurse and medical team regarding the patient’s condition and goals of care, and
- astute observations of the patient.
It has not been tested, yet, but you may find that this is already what is going on inside of your critically-thinking SLP brain! Now, we all need YOU to comment, question, and edit the tool. Please continue this conversation below.
I look forward to further research on this important topic as there is a significant urgency to ensure safer oral intake when people are on high-flow nasal cannula devices, especially now in the era of COVID-19.
In discussing the future with Katie Gersbach Allen (of Allen & Galek, 2020 research), she reiterated (in personal communication Dec 8, 2020):
“There’s so much still to learn about high-flow nasal cannula (HFNC) and swallowing. It is so tough to know yet exactly what to consider when guiding clinicians. However, it’s vital for clinicians to look at swallow physiology with patients on HFNC, namely kinematics/timing. The PAS scores are not sensitive enough to capture the subtle changes in the laryngeal vestibule during HFNC.”
Guidelines for Planning PO Intake for People on High-Flow Nasal Cannula (HFNC)
by Karen Sheffler, MS, CCC-SLP, BCS-S of SwallowStudy.com (December, 2020)
| QUESTIONS (YES / NO) | CONSIDERATIONS | |
| 1. Stable respiratory status on HFNC? | Discussion with nurse & medical team: meaning not risk for a downgrade to CPAP, BiPAP or intubation for mechanical ventilation | |
| 2. Lower airflow rate? | 40 liters per minute and above may be cause for increased caution. Increased risk of aspiration, variability, and difficulty swallowing due to high airflow pressures in the nasopharynx (close to or at CPAP levels). | |
| 3. FiO2 not near 100%? | If someone is on an FiO2 of 99-100%, that indicates that they are unstable and rates this high are usually for 24 hours or less. | |
| 4. Respiratory rate less than 25 – 30? | Under 30 breaths per minute. Above 25 breaths per minute should raise concerns. | |
| 5. Stable medical condition? | Per discussion with nurse and medical team. | |
| 6. Improving physical strength and condition? | Are they ambulatory (independent or with assistance)? Not bedridden? Will have improved mucociliary clearance and pulmonary clearance if they have trace aspiration.
Medical fragility, frailty and sarcopenia would increase risks for dysphagia and aspiration. |
|
| 7. Cleared for oral intake per the medical team? | Discuss thoroughly with nurse(s) and medical team. Risks/benefits of attempting PO trials. | |
| 8. Adequate mental status to participate at mealtimes with supervision and/or assistance? | How is their attention to task? No agitation, distractibility, impulsiveness? Eng, et al. (2019) and Flores, et al. (2019) noted that the degree of cognitive impairment was a big part of decision-making. | |
| 9. Generally orientated? | Knows name. At least generally oriented to place and time. Person with lack of orientation has higher odds of aspirating. (Leder, et al., 2009) | |
| 10. Follows 1-step commands? | At least able to participate in oral sensorimotor examination, such as stick out your tongue and smile. Inability to follow commands has higher odds of aspiration. (Leder, et al., 2009) | |
| 11. Adequate tongue range of motion? | Impaired lingual range of motion was associated with aspiration (Leder, et al., 2013) | |
| 12. Able to manage own secretions without significant suctioning? | Discuss with nurse; observe and listen to patient | |
| 13. Passes the 3-ounce water screen portion of the Yale Swallow Protocol or similar swallow screen? | PASS: drinking 3 ounces of water with consecutive sip/swallows – without stopping.
FAIL: inability to drink in a continuous fashion, stopping, throat clearing, coughing immediately or in delay, distress, or significant change to a wet-gurgly vocal quality |
|
| 14. Adequate oral hygiene and a routine of good oral decontamination/oral infection control? | Review the importance of thorough mouth, tongue, and teeth brushing with a toothbrush and mouthwash; and not simply swabbing with toothettes. Prevents aspiration pneumonia. | |
| TOTAL YES answers: _____ out of 14.
|
If the number is close to 14, you may be more confident to attempt your first trials of foods and liquids by mouth at the bedside. Watch how the person’s work of breathing and respiratory-swallowing coordination change with sitting up and eating/drinking. Perform an instrumental evaluation if any concerns.
If there is a lower number, in addition to overt signs/symptoms of aspiration, and other concerns: Consider NPO. The person may need more time and/or an instrumental evaluation prior to further oral intake decision-making. |
|
| Note and consider the following, but these may not necessarily be exclusionary factors. | ||
| Gender? | Females may have higher expiratory pressures | |
| Age? | Younger and healthier subjects in studies seem to show adaptation to high pressures in the short-term | |
| How long have they been on HFNC? | Longer-term use may desensitize the area. The airflow may initially heighten awareness of the region, this airflow and pressures may eventually desensitize and potentially decreased the sensation of residue and aspiration. | |
| What are the person’s (healthcare proxy’s/HCP) stated goals of care and amount of accepted risk for aspiration and choking? These can lead to aspiration pneumonia, a decline in medical condition, and death? | Healthcare professionals will document all discussions with the person (and HCP) regarding goals of care, decision-making, and wishes surrounding the risks/benefits of initiating any oral intake. | |
| NOTE | This chart is paired with information and references in this blog: High-Flow Nasal Cannula (HFNC): Does it increase dysphagia & aspiration risk?
https://swallowstudy.com/high-flow-nasal-cannula-hfnc-does-it-increase-dysphagia-aspiration-risk/ |
|
| Draft 12/2020 | Print PDF: Guidelines for Planning PO Intake for People on HFNC | |
Thank you for making it to the end! Never stop questioning!
*******
NEW Pediatric resource for High-Flow Nasal Cannula (HFNC)
Thank you to Sheri Rosen, MA, CCC-SLP, Lead Speech Pathologist at UPMC Children’s Hospital of Pittsburgh shared this pdf in July 2022.
References for High-Flow Nasal Cannula
Allen, K. & Galek, K. (2020). The influence of airflow via high-flow nasal cannula on duration of laryngeal vestibule closure. Dysphagia, published online https://doi.org/10.1007/s00455-020-10193-0
Alshuwaikhat, H., Scott, B. & LaGorio, L. (2020, October). The Impact of High-Flow Nasal Cannula on Swallow Function. Respiratory Care, 65 (Issue Suppl 10) 3440132. https://rc.rcjournal.com/content/65/Suppl_10/3440132 — only available in this abstract form
Coghlan, K., & Skoretz, S. (2017). Breathing and swallowing with high flow nasal cannula therapy. Perspectives of the ASHA Special Interest Groups, 2(3), 74–81.
Coyle, J. L. (2010). Ventilation, respiration, pulmonary diseases, and swallowing. SIG 13 Perspectives on Swallowing and Swallowing Disorders (Dysphagia), 19(4), 91–97.
Crary, M.A., Sura, L., Carnaby, G. (2013a). Validation and demonstration of an isolated acoustic recording technique to estimate spontaneous swallow frequency. Dysphagia, 28(1), 86-94. doi: 10.1007/s00455-012-9416-y. Epub 2012 Jun 17. PMID: 22707084.
Crary, M.A., Carnaby, G., Sia, I., Khanna, A., Waters, M.F. (2013b). Spontaneous swallowing frequency has potential to identify dysphagia in acute stroke. Stroke, 3452-3457. DOI: 10.1161/STROKEAHA.113.003048
Cvejic, L., Harding, R., Churchward, T., Turton, A., Finlay, P., Massey, D., Bardin, P.G., Guy, P. (2011). Laryngeal penetration and aspiration in individuals with stable COPD. Respirology, 16(2), 269-75. doi: 10.1111/j.1440-1843.2010.01875.x. PMID: 21054669.
Eng, K., Flores, M. J., Gerrity, E., Sinha, N., Imbeau, K., Erbele, L. & Yeh, C. (2019, December). Evaluation of Swallow Function on Healthy Adults While Using High-Flow Nasal Cannula. Perspectives of the ASHA Special Interest Groups, 4, 1516-1524. Downloaded from: https://pubs.asha.org
Flores, M. J., Eng, K., Gerrity, E., & Sinha, N. (2019). Initiation of oral intake in patients using high flow nasal Cannula: A retrospective analysis. Perspectives of the ASHA Special Interest Groups, 4, 522–531. Downloaded from: https://pubs.asha.org
Fraser, J.F., Corley, A., Caruana, L.R., Tronstad, O., Barnett, A.G. (2010). Nasal high flow oxygen increases end expiratory lung volumes, improves oxygenation and reduces work of breathing: a study using electrical impedance tomography. Am J Respir Crit Care Med, 181(Suppl), A1668. (Recommended in Parke, 2011 article re inhalation versus exhalation process/pressures)
Gotera, C., Díaz Lobato, S., Pinto, T., Winck, J.C. (2013). Clinical evidence on high flow oxygen therapy and active humidification in adults. Rev Port Pneumol, 19, 217-227.
Groves, N. & Tobin, A. (2007). High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care, 20(4), 126-31. doi: 10.1016/j.aucc.2007.08.001. Epub 2007 Oct 10. PMID: 17931878. (regarding female’s pressures are higher pressure in Parke, et al., 2013 summary)
Hernandez, G., Vaquero, C., Gonzalez, P., Subira, C., Frutos-Vivar, F., Rialp, G., . . . Fernandez, R. (2016). Effect of post-extubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: A randomized clinical trial. Journal of the American Medical Association, 315(13), 1354–1361. https://doi.org/10.1001/jama.2016.2711
Keenan, S.P., Kernerman, P.D., Cook, D.J. (1997). Effect of noninvasive positive pressure ventilation on mortality in patients admitted with acute respiratory failure: A meta-analysis. Crit Care Med, 25, 1685 – 92.
Leder, S.B., Suiter, D.M. & Warner, H.L. (2009). Answering orientation questions and following single-step verbal commands; Effect on aspiration status. Dysphagia, 24, 290-295.
Leder, S.B., Suiter, D.M. (2014). The Yale swallow protocol: An evidenced-based approach to decision making. New York: Springer, 15.
Leder, S.B., Suiter DM, Warner HL, Kaplan LJ. (2011). Initiating safe oral feeding in critically ill intensive care and step-down unit patients based on passing a 3-ounce (90 milliliters) water swallow challenge. J Trauma, 70, 1203–7.
Leder, S.B., Suiter, D.M., Murray, J. & Rademaker, A.W. (2013). Can an oral mechanism examination contribute to the assessment of odds of aspiration? Dysphagia, 28, 370-374.
Leder, S. B., Siner, J. M., Bizzarro, M. J., McGinley, B. M., & Lefton-Greif, M. A. (2016). Oral alimentation in neonatal and adult populations requiring high-flow oxygen via nasal cannula. Dysphagia, 31(2), 154–159.
Martin-Harris, B. (2008). Clinical implications of respiratory-swallowing interactions. Current Opinion in Otolaryngology & Head and Neck Surgery, 16(3), 194–199. https://doi.org/10.1097/MOO.0b013e3282febd4b
Martin-Harris, B., Brodsky, M. B., Michel, Y., Ford, C. L., Walters, B., & Heffner, J. (2005). Breathing and swallowing dynamics across the adult lifespan. Archives of Otolaryngology–Head and Neck Surgery, 131(9), 762–770. https://doi.org/10.1001/archotol.131.9.762
McFarland, D. H., Martin-Harris, B., Fortin, A. J., Humphries, K., Hill, E., & Armeson, K. (2016). Respiratory- swallowing coordination in normal subjects: Lung volume at swallowing initiation. Respiratory Physiology & Neurobiology, 234, 89–96. https://doi.org/10.1016/j.resp.2016.09.004
Molfenter, S.M. & Steele, C.M. (2012). Temporal variability in the deglutition literature. Dysphagia, 27, 162–77.
Nishimura, M. (2016). High-flow nasal cannula oxygen therapy in adults: Physiological benefits, indication, clinical benefits, and adverse effects. Respiratory Care, 61(4), 529–541. https://doi.org/10.4187/respcare.04577
Oomagari, M., Fujishima, I., Katagiri, N., Arizono, S., Watanabe, K., Ohno, T., et al. (2015). Swallowing function during high-flow nasal cannula therapy. European Respiratory Journal, 46 (suppl 59) PA4199; DOI: 10.1183/13993003.congress-2015.PA4199 https://erj.ersjournals.com/content/46/suppl_59/PA4199— only available in this abstract form
Parke, R.L., McGuinness, S.P., & Eccleston, M. (2009). Nasal high-flow therapy delivers low level positive airway pressure. British Journal of Anaesthesia, 103(6), 886-890.
Parke, R.L., McGuinness, S.P., & Eccleston, M. (2011). A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respiratory Care, 56 (3), 265-270.
Parke, R. L., & McGuinness, S.P. (2013). Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care, 58(10), 1621-1624.
Roca, O., Hernandez, G., Diaz-Lobato, S., Carratala, J.M., Gutierrez, R.M., Masclans, J.R., & Spanish Multidisciplinary Group of High Flow Supportive Therapy (2016). Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Critical Care, 20(1), 109. https://doi.org/10.1186/s13054-016-1263-z
Rosenbek, J.C., Robbins, J., Roecker, E.B., Coyle, J.L., Wood, J.L. (1996). A penetration-aspiration scale. Dysphagia, 11, 93–8.
Sanuki, T., Mishima, G., Kiriishi, K. et al. Effect of nasal high-flow oxygen therapy on the swallowing reflex: an in vivo volunteer study. Clin Oral Invest 21, 915–920 (2017). https://doi.org/10.1007/s00784-016-1822-3
Singer, P. & Rattanachaiwong, S. (2018). To eat or to breathe? The answer is both! Nutritional managment during noninvasive ventilation. Critical Care, 22, 27. DOI 10.1186/s13054-018-1947-7
Terzi, N., Darmon, M., Reignier, J., Ruckly, S., Garrouste-Orgeas, M., Lautrette, A., … & Timsit, J.F. OUTCOMEREA study group. (2017). Initial nutritional management during noninvasive ventilation and outcomes: A retrospective cohort study. Crit Care, 21(1), 293. doi: 10.1186/s13054-017-1867-y. PMID: 29187261; PMCID: PMC5707783.
Ward, J.J. (2013). High-flow oxygen administration by nasal cannula for adult and perinatal patients. Respiratory Care, 58(1), 98-122.


This blog post is jam-packed full of the evidence we currently know (as of December 2020!) and captures the shortcomings in published research on HFNC and swallowing disorders. The discussion-oriented format of your post leads naturally to a clinically accessible tool that needs to be included in a research study! I am so glad you took the time to put this blog post and the tool.
My only caution/advice is that SLPs who use the proposed tool or any other methods of clinical swallow evaluation should be cautioned to be critical appraisers of the evidence when it comes to “PO trials” with this population (HFNC, respiratory compromise, critically ill), and with clinical swallowing evaluations in general. We know that our clinical exam is limited and we require imaging to evaluate pharyngeal Dysphagia. It would be unfortunate to see someone thinking they can trial thickened liquids at the bedside, when thickened liquids are presumably an intervention for pharyngeal Dysphagia (and, as noted, pharyngeal Dysphagia can only be evaluated with imaging), and subsequently recommending a modified diet without the use of an instrumental exam.
Our bar for “concerned for Dysphagia” should be very low with the critically ill. When we identify possible Dysphagia during the clinical exam and let that guide us to appropriate use of instrumental exams, we enable the patient to have the most appropriate treatment (or no treatment) for Dysphagia. And NOW, we have another fabulous resource to guide our thinking during the clinical swallow evaluation.
Bravo!
Exactly! Thank you for this! I edited the beginning section to classify the framework further.
The “tool” as you say is really a framework, as I noted that it is not at all trialed on the frontlines or in research. Since I wanted to make a list of guidelines by the end of the blog, it came together nicely as an easy to read chart. See new pdf also. I hope I made it very clear, also, that after answering the 14 questions during your info gathering, that the process has just given you a bit more “confidence” in attempting a small amount of PO at bedside. Clinicians should err on the side of caution if at all under 14. Even if the patient reaches 14 out of 14! Using an instrumental is really essential for these patients. That was the sentiment of Eng, Flores, and Allen & Galek researchers as well. With instrumental evaluations we can start teasing out what changes in these critically ill patients under the presence of high airflows and high pressures.
Ultimately, I hope the chart frames your questions to the team better, as it does NOT give the answers. It frames your thinking to err on side of caution with these more respiratory fragile patients. The chart and your answers should not automatically place a person on a diet without careful evaluation and hopefully a low bar to move to instrumental evaluation to: fully define dysphagia, its severity, its pathophysiology, what we can do about it in treatment, what strategies are useful, and what diet is “safer.” You can even ask the person during your VFSS or FEES what they are feeling in the presence of the airflow — do they feel the residue you are seeing; do they feel the airway invasion? Testing sensation in these folks will be critical.
Thought-provoking, practical, and comprehensive. This review of the literature and the new insights you have provided is going to change the way I practice. Thank you so much for this contribution to the field!