Path to a Dysphagia Protocol for People Post-Lung Transplant
by Sarah Russell, MS, CCC-SLP, BCS-S
Editor & Co-author: Karen Sheffler, MS, CCC-SLP, BCS-S of SwallowStudy.com
It was early 2020. (Remember that time just before COVID-19 hit?) I sat in my office with a PowerPoint presentation, anxious to log on to the joint Thoracic Surgery and Transplant Pulmonology educational meeting where I was asked to present on aspiration risk in our post-lung transplant population. I’m not sure if you have ever presented to a group of Thoracic Surgeons before, but I was slightly intimidated, and I have a lot of letters after my name and 20+ years of experience as a medical speech pathologist specializing in difficulty swallowing (dysphagia).
Path to a Post-Lung Transplant Dysphagia Protocol
I had previously passed along to one of the surgeons the Baumann and colleagues article from 2017 titled: “Postoperative Swallowing Assessment After Lung Transplantation.” Since that article was so compelling, they were willing to listen and invited me to lecture.
Briefly, Brooke Baumann and colleagues from Pittsburgh and Iowa City retrospectively studied all people post-lung transplant from 2009 to 2012. They found penetration to the level of the vocal cords or aspiration in 198/297 (67%) of patients tested with instrumental examinations. These occurred silently in 184 out of 198 participants (62%), meaning they had no obvious sensory response at the bedside. Even a subgroup of 81 people who were deemed to have a normal bedside clinical examination, actually had this deep penetration to the vocal cords or aspiration. The researchers recommended that everyone should receive a temporary feeding tube through the nose within the operating room and have an instrumental examination prior to oral intake (e.g., Fiberoptic Endoscopic Evaluations of Swallowing/FEES or a Modified Barium Swallow Study/Videofluoroscopic Swallow Study/VFSS).
I was ready for the presentation with multiple FEES recordings and the few prior research papers on oropharyngeal dysphagia in this population. Yes, there are only a few. See references below. For example, Atkins and colleagues in 2007 noted “strikingly high rates of dysphagia” and aspiration risk that increased morbidity in people post-lung transplant. They noted penetration and/or aspiration in 70.5% of people using FEES or VFSS. An alarming 77.6% of those were silently occurring (without any outward signs).
A Picture Speaks a Thousand Words
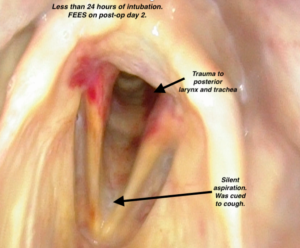
This person was intubated for less than 24 hours. This FEES was performed 2 days after a bilateral lung transplant.
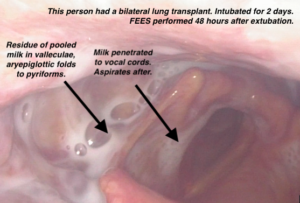
Significant penetrations to the level of the vocal cords that are not ejected will fall into the trachea in a silent aspiration after the swallow.
Same person post-lung transplant (bilateral). Attempts to cough the milk out of the laryngeal vestibule were not effective.
The FEES video images had a huge impact, as I showed examples of people who had silent aspiration from a variety of scenarios: after a single lung transplant, after an uncomplicated bilateral lung transplants, and even after intubations of less than 24 hours.
The surgeons and other physicians watched frame after frame of milk or blue water sliding into the trachea without so much as a single cough. There were audible gasps in the audience, especially from the pulmonologists.
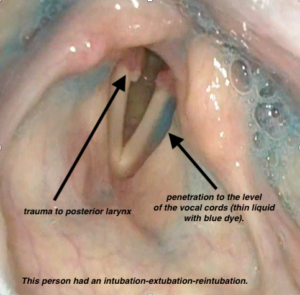
increased trauma to the posterior larynx
“What do we need to do?” the medical team asked.
My solution was for speech-language pathologists (SLPs) to be consulted for a swallowing evaluation for every person who had undergone a lung transplant after extubation. I advised that all patients would receive formal instrumental swallowing evaluations (FEES) prior to any oral (PO) intake, including prior to any oral medications.
I was expecting a bunch of excuses and reasons why that would not be possible, but to my surprise they just said: “OK.”
Sometimes research does not directly translate into patient care, but in this case, just that one Baumann article and a few video images had a dramatic effect. I am so grateful and send a huge thank you to all of the researchers out there putting data together and publishing for us clinicians in the trenches!
Collaborations
After a brief pause at the onset of the COVID-19 pandemic, I got back to collaborating with our cardiothoracic unit nurse clinical specialists and nurse educators to formulate a protocol. We developed and rolled out the education to all the pertinent staff members. This was not a seamless adventure as there are many layers of red tape any time new protocols are established in a large university teaching hospital. By the end of 2020, we had a protocol in place we could all agree on. The nurse practitioners, physician’s assistants, registered nurses (RNs), and critical care intensivists all knew about the protocol.
Imaging Needed on All People Post-Lung Transplant
When the consults rolled in, we started every patient with a bedside/clinical swallowing evaluation. This helped to determine the timing of the instrumental evaluation. Typically a FEES is performed the day after extubation, but timing is dependent on factors such as:
- alertness,
- pain control, and
- breathing comfortably with oxygen demands less than 6 liters via nasal cannula.
Did we really need to do a FEES on EVERY patient? The answer so far is YES.
Are there certain patient characteristics that would allow a person who is post-lung transplant to slide by without imaging? The answer so far is NO.
I have not yet identified a certain pattern that would suggest no suspected risk for aspiration. No patterns noted regarding: patient population, type of surgery, or intubation duration to suggest no risk. Therefore, we continue to formally image every patient.
There is some nuance to all of this, however. The art of medicine, I suppose. Right after we started the protocol, I would anxiously track each person’s post-lung transplant course, looking for when the pain service placed an epidural, if appropriate. I scanned the flowsheets for extubation so I could pounce on the patient. I would then introduce myself and kindly ask them if I could put a small-flexible camera through their nose to look at their swallowing. “Don’t worry, you won’t feel a thing.”
By the way, our surgeons did not want to place dobhoff feeding tubes in the operating room because they often get dislodged with extubation. Therefore, I worried that the RNs would be waiting for my swallowing evaluation in order to give the mountain of immunosuppressant medications now needed for this transplanted organ.
Tracking Dysphagia Patterns Post-Lung Transplant
I kept track of post-lung transplant dysphagia-related outcomes, underlying diagnoses, intubation duration, any laryngeal findings, dysphagia characteristics, and diet textures recommended in search for patterns.
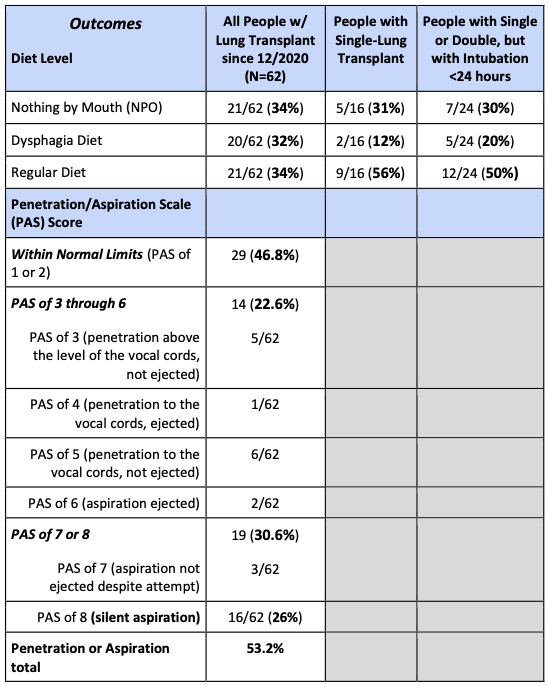
Preliminary data from Michigan Medicine, per Sarah Russell, MS, CCC-SLP, BCS-S, gathered after initiating the swallowing/dysphagia protocol for people post-lung transplant. Data is based on the first FEES examination, which is typically 1 day after extubation.
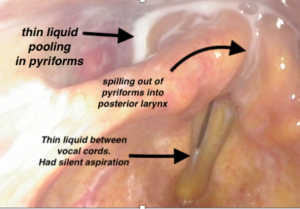
This is an example of trace & silent aspiration. Seen here between the vocal cords prior to falling below the vocal cords in an aspiration. When aspiration is silent with no effort made to eject or cough it out, that is a PAS score of 8 (out of 8).
Those people who remained NPO after their first examination (34% in above chart) had the following characteristics:
- 17/21 people had diagnosis of Idiopathic Pulmonary Fibrosis (IPF)/ Interstitial Lung Disease (ILD)
- Average length of intubation*: 4.5 days (5/21 needed re-intubations)
- Average age: 55 years old
- 3/21 needed trach placements
- Longer time to return to a regular diet (only 4/21 resumed a regular diet after repeat imaging)
- Pharyngeal residue and vocal fold hypomobility were common
*It must be highlighted that intubation for this complex population is not only the duration of intubation post-lung transplant surgery, but also the size of the lumen. During surgery, the person has a double lumen tube (larger) for the endotracheal and endobronchial channels. Additionally, there is often a transesophageal echocardiogram (TEE) probe in place for the duration of the operation. Read more about tracheal tube sizes and TEE risk factors in Dr. Emily Plowman and team’s work (2023).
Observations of the preliminary characteristics of the pharyngeal dysphagia:
- Pharyngeal residue due to suspected pharyngeal weakness, even in those intubated for only 48 hours.
- Residue pooling in the pyriform sinuses tends to spill forward into the posterior laryngeal vestibule (see photos above). This is suspected to be due to reduced hyolaryngeal excursion and/or cricopharyngeal dysfunction.
- Mistiming of the laryngeal vestibule closure/LVC (i.e., slow-to-LVC action), which may be due to a sensory impairment from superior laryngeal nerve impairment.
- Incomplete laryngeal vestibule closure (as seen in the videofluoroscopic swallow study image below).
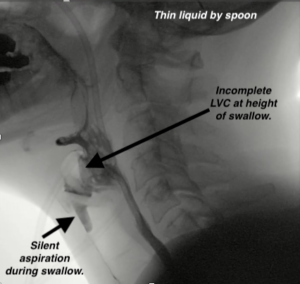
Videofluoroscopic Swallow Study/VFSS, aka, Modified Barium Swallow Study/MBSS performed 3 weeks post-lung transplant due to multiple intubations (one being a 1-week intubation) and other complications. This person had no response to this large volume of aspiration. That is a silent aspiration (PAS 8). One motoric underlying etiology pattern was: reduced hyolaryngeal excursion & reduced tongue base propulsion, causing reduced bolus pressure and reduced epiglottic inversion. This pattern causes the incomplete laryngeal vestibule closure seen here. Therefore, airway protection is reduced. When that is combined with reduced sensation, there is a high risk for ongoing aspiration.
Frequent laryngeal findings are:
- True vocal fold hypomobility (reduced movement) or immobility (no movement). The SLP describes the reduced movement or bowing characteristics. The Otolaryngologist will define or diagnose with paresis or paralysis and find the underlying causes (e.g., trauma versus recurrent laryngeal nerve injury);
- Arytenoid edema;
- Airway redness and bruising.
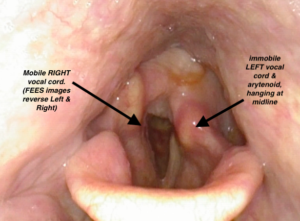
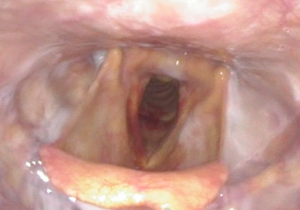
This person had a bilateral lung transplant and was intubated for 3 days. This FEES image shows that the person’s right side (left side of photo above) is abducting (opening) well, but the left side is hanging at midline immobile (aka, paramedian position). The SLP identifies this unilateral Left true vocal cord immobility, while the Otolaryngologist is the one to diagnose with paralysis.
More Lessons Learned
Now that I have been doing this for some time and I know those immunosuppressants can be given IV, I do not rush to complete that first swallow study. I am also now armed with data that each person post-lung transplant has about a 30% chance of having a normal looking swallow on that first exam. I also have observed the characteristics of those who tend to still require increased caution and an NPO (nothing by mouth) order after their first FEES. Those warning signs, as noted above, have been:
- Average length of intubation of 4.5 days,
- Re-intubations and the need for tracheostomy placements,
- Increased amounts of residue noted on first instrumental examination, and
- Findings of vocal fold hypomobility.
Literature has so far found the following risk factors for post-lung transplant oropharyngeal dysphagia and aspiration (Atkins, Trachtenberg et al., 2007; Atkins, Peterson et al., 2010; Plowman et al, 2023):
- Advanced age,
- Pre-operative gastroesophageal reflux (GERD),
- Need for cardiopulmonary bypass intraoperatively,
- Renal failure,
- ECMO, and
- Re-intubation.
Increased time can be a friend
SLPs also know that time can be our friend and waiting until the next day is often beneficial for a less restrictive diet (see research by Marvin et al., 2019). It is important for the SLP to discuss the risks and benefits and to make plans with the whole team. Sometimes we need to prioritize the following prior to pushing for oral intake:
- pain management,
- practicing breathing with their new lungs, and
- allowing the sedating medications to wear off after extubation.
Fortunately, most of the people I have cared for have resolved their dysphagia issues by the time they are discharged home, and many have spontaneous improvement within 3-5 days. I postulate that may be due to their improving laryngeal sensation with time, among other factors.
More Questions than Answers
As I continue caring for this patient population and tracking data, more questions than answers have surfaced.
The age old question: How much aspiration is too much?
As SLPs, we know that many people aspirate without consequence, while others develop aspiration pneumonia. See part 1 and part 2 of this SwallowStudy.com article regarding types of pneumonia (e.g., is it a dysphagia-related aspiration pneumonia?). See also the important “Three Pillars of Aspiration Pneumonia,” coined by Dr. John R Ashford. We consider the three pillars of:
- Aspiration due to impaired airway protection: We ask about the volume of aspiration and frequency of aspiration. Dr. Harmeet Singh Bedi, of Stanford University, discussed this at the Dysphagia Research Society’s Annual Meeting in March 2023 in San Francisco at the March 17th invited session titled: “Focus on Pulmonary Function and Disorders.” He noted: Why, when, and how much aspiration are significant questions and big lung factors (i.e., macro issues versus micro aspiration and the frequency over time). However, Dr. Bedi indicated that we still don’t know how much is too much. It is likely very patient-specific.
- Contents of the aspiration: We need to know if it is from:
- prandial aspiration (during meal or any oral intake) that involves pathogenic oral bacteria from a dirty oral environment (aka, oral flora or biofilm) or
- reflux aspiration that could cause pneumonitis, at least (chemical injury/irritation/inflammation).
- Host immunity: The third pillar asks if the person has the ability to fight off infection. Those who are post-lung transplants, who require immunosuppressant medications, will have a harder time fighting off infection.
With FEES we can see trace amounts of aspiration. I am exceedingly conservative with the people I work with who have new lungs, but we do not have great data on whether trace prandial aspiration of food or drink ultimately contributes to allograft failure. However, this patient population has an estimated 5-year survival rate of 50-60% (85% one-year survival rate) due to the fragility of transplanted lungs. Therefore, I cannot confidently say that trace aspiration does not matter.
We do know reflux aspiration contributes to graft failure. In people who are supine, the most common gravity dependent area for reflux aspiration will be the posterior segment of the right upper lobe, per Dr. Bedi’s talk. Many patients with end-stage lung disease have baselines of GERD prior to their lung transplant (i.e., gastroesophageal reflux disease with risks for supraesophageal reflux). Post-transplant reflux continues to be an issue. Bile acids were found in the lungs of about half of 50 lung transplant recipients (D’Ovidio et al., 2005 & 2006), and they noted that aspiration of this refluxant was associated with post-lung transplant bronchiolitis obliterans syndrome (BOS). Bile acid aspiration is associated with an increased rate of allograft dysfunction and decreased survival (Blondeau et al., 2008). See also Atkins and colleagues’ 2007 and 2010 studies that noted pre-operative gastroesophageal reflux was an independent predictor of post-transplant oropharyngeal dysphagia.
Dr. Walter W. Chan, MD, MPH (Division of Gastroenterology, Hepatology and Endoscopy from Brigham & Woman’s Hospital, Boston) also spoke at the March 2023 Dysphagia Research Society Annual Meeting in a session titled: “Foregut Airway Interactions.” See his review article with colleagues (Hathorn et al., 2017) on how GERD affects the outcomes of lung transplantation. During his talk, he discussed the bidirectional relationship of lung disease and GERD/esophageal dysmotility. As noted above, GERD can cause lung injury from acute to chronic reflux aspirations. He added that people with ineffective esophageal motility (IEM) have been found to be more likely to reject the transplanted lung, independent of the reflux issue (Wong & Chan, 2021). In the other direction, restrictive lung disease can cause increased thoraco-abdominal pressures that can increase reflux. In a more hopeful side of this bidirectional relationship, he noted that the improvement to lung function achieved with lung transplantation can allow for the improved contractile forces within the esophagus and for reflux to diminish. This is a cursory review of Dr. Chan’s very informative lecture, but we suggest selected references below and searching Dr. Walter W. Chan in PubMed for his extensive research in lung transplantation from a GI standpoint.
See other educational opportunities through the Dysphagia Research Society at their website and
through the Dysphagia Research Society’s Institute for Education (DRSIE).
More Questions
As I said, I have more questions now than answers, and you may have these same questions, such as:
- Do I have a protocol established for the optimal timing for repeat imaging after the initial imaging shows an abnormal swallow? Not yet.
- Should all patients just get a feeding tube for a few days and have their initial FEES 3 days after extubation to give more time for recovery? The 30% of patients with a normal swallow who could have been eating and drinking for 3 days would likely take issue with that plan.
- What are the optimal treatment strategies for this patient population?
- Should everyone be started on a program of swallowing exercises BEFORE the lung transplantation?
- Is there a magical unicorn patient population that will not have post-op dysphagia?
- Will technology advance so there is less risk of laryngeal trauma from intubations and nerve manipulation during surgery?
Tips on Instrumental Evaluations in People Post-Lung Transplant:
- FEES is my preferred first study post-transplant to get a good view of any trauma to the larynx. It is portable, keeping the complex ICU patients in the ICU. You can test oral medications with a FEES to give updated recommendations to the Transplant Pharmacist.
- FEES is great for early biofeedback and education. When patients can see residue or aspiration they have a better understanding why food or drink may be withheld. Most lung transplant patients desperately want to protect their new lungs at all costs.
- When performing a videofluoroscopic swallow study (VFSS), make sure to test the barium pill with esophageal sweep. (Of course, this would be as tolerated and per clinician discretion per the individual’s abilities). Keep in mind that many of the anti-rejection transplant medications cannot be crushed. Even if you tested this pre-transplant, test again post-transplant. You may see an improvement in esophageal function post-transplant compared with findings on their pre-op esophagram. The VFSS with esophageal sweep is in line with the findings noted above that many people with impaired lung function also have impaired esophageal motility. The SLP makes appropriate referrals and gives safer eating/drinking strategies based on these VFSS findings.
- Make detailed recommendations based on your instrumental exam to guide therapy and provide a timeline for repeat instrumental testing for safe diet advancement. Many people make significant progress in the matter of days. I often repeat imaging within 3-4 days of the first test.
- Provide hope: Even when significant caution is required for some, other people often can resume a regular diet without restrictions within 3 months. The detailed guidance from the SLP keeps the person safer for best possible eating/swallowing outcomes.
Post-Lung Transplant & Dysphagia Summary
We must keep in mind that each person comes to the operating table with a list of premorbid conditions (aka, past medical history) that may impact their recovery. Therefore, we continue to provide person-centered and evidence-based care, including considering the whole person and their goals and wishes.
So far, since our swallowing/dysphagia protocol started, there have been 0 cases of CLAD (chronic lung allograft dysfunction – i.e., bronchiolitis obliterans syndrome or restrictive allograft syndrome). It is early on in the life of the protocol, but Michigan Medicine remains dedicated to this collaborative protocol.
With that, I implore our dysphagia researchers to continue answering these questions and sharing their findings with us clinicians. The researcher-clinician dynamic is a two-way street, as the clinicians in the trenches need to continue to ask hard questions and seek answers for the people they serve. That feeds research. My hope is to continue to share and collaborate as we learn more about this complicated population of people with acquired dysphagia status post-lung transplant.
Thank you to my Guest Writer:
Sarah Russell, MS, CCC-SLP, BCS-S
 Sarah graduated in 2001 with a Master’s of Science in Communication Sciences and Disorders from the MGH Institute of Health Professions at Massachusetts General Hospital in Boston. She worked at Advocate Lutheran General Hospital in Park Ridge, IL for seven years. In 2011, Sarah moved back to Boston to work at Brigham and Women’s Hospital, where she managed a variety of complex dysphagia cases. Sarah has been practicing at Michigan Medicine for nearly ten years. Her passion is managing dysphagia in critically ill patients in the intensive care unit, specifically following cardiothoracic surgery. Sarah achieved Board Certification in Swallowing and Swallowing Disorders in 2014. She is the organizer of the Michigan Medicine Dysphagia Grand Rounds, where clinicians attend to learn about evidence-based management of dysphagia. You can email her at srusse@med.umich.edu for more information and to be put on the email list for the Grand Rounds series.
Sarah graduated in 2001 with a Master’s of Science in Communication Sciences and Disorders from the MGH Institute of Health Professions at Massachusetts General Hospital in Boston. She worked at Advocate Lutheran General Hospital in Park Ridge, IL for seven years. In 2011, Sarah moved back to Boston to work at Brigham and Women’s Hospital, where she managed a variety of complex dysphagia cases. Sarah has been practicing at Michigan Medicine for nearly ten years. Her passion is managing dysphagia in critically ill patients in the intensive care unit, specifically following cardiothoracic surgery. Sarah achieved Board Certification in Swallowing and Swallowing Disorders in 2014. She is the organizer of the Michigan Medicine Dysphagia Grand Rounds, where clinicians attend to learn about evidence-based management of dysphagia. You can email her at srusse@med.umich.edu for more information and to be put on the email list for the Grand Rounds series.
References from Sarah Russell:
- https://www.uofmhealth.org/conditions-treatments/transplant/lung-transplant-process
- https://www.uptodate.com/contents/lung-isolation-techniques#H3404936359
- U-M Health Transplant Center: Pre-Transplant Education for Lung Transplantation
Atkins BZ, Trachtenberg MS, Prince-Petersen R, et al. Assessing oro-pharyngeal dysphagia after lung transplantation: altered swallowing mechanisms and increased morbidity. Journal of Heart and Lung Transplant. 2007;26:1144-8. https://pubmed.ncbi.nlm.nih.gov/18022080/
Atkins BZ, Petersen RP, Daneshmand MA, Turek JW, Lin SS, DavisRD. Impact of oropharyngeal dysphagia on long-term outcomes of lung transplantation. Ann Thorac Surg 2010;90:1622-8. https://pubmed.ncbi.nlm.nih.gov/20971276/
Baumann B, Byers S, Wasserman-Wincko T, et al. Postoperative swallowing assessment after lung transplantation. Ann ThoracSurg 2017;104:308-12. https://pubmed.ncbi.nlm.nih.gov/28483151/
Black R, McCabe P, Glanville A, Bogaardt H, MacDonald P, Madill C. Oropharyngeal dysphagia and laryngeal dysfunction after lung and heart transplantation: A systematic review. Disabil Rehabil. 2020 Jul;42(15):2083-2092. doi: 10.1080/09638288.2018.1552326. Epub 2019 Jan 29. PMID: 30694075. https://pubmed.ncbi.nlm.nih.gov/30694075/
Blondeau K, Mertens V, Vanaudenaerde BA, Verleden GM, Van Raemdonck DE, Sifrim D, Dupont LJ. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008 Apr;31(4):707-13. doi: 10.1183/09031936.00064807. Epub 2007 Dec 5. PMID: 18057058. https://pubmed.ncbi.nlm.nih.gov/18057058/
D’Ovidio F, Mura M, Tsang M, Waddell TK, Hutcheon MA, Singer LG, Hadjiliadis D, Chaparro C, Gutierrez C, Pierre A, Darling G, Liu M, Keshavjee S. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005 May;129(5):1144-52. doi: 10.1016/j.jtcvs.2004.10.035. PMID: 15867792. https://pubmed.ncbi.nlm.nih.gov/15867792/
D’Ovidio F, Mura M, Ridsdale R, Takahashi H, Waddell TK, Hutcheon M, Hadjiliadis D, Singer LG, Pierre A, Chaparro C, Gutierrez C, Miller L, Darling G, Liu M, Post M, Keshavjee S. The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006 Aug;6(8):1930-8. doi: 10.1111/j.1600-6143.2006.01357.x. PMID: 16889547. https://pubmed.ncbi.nlm.nih.gov/16889547/
Duarte AG, Terminella L, Smith JT, Myers AC, Campbell G, Lick S. Restoration of cough reflex in lung transplant recipients. Chest. 2008 Aug;134(2):310-316. doi: 10.1378/chest.07-2934. Epub 2008 Mar 13. PMID: 18339778. https://pubmed.ncbi.nlm.nih.gov/18339778/
Plowman EK, Anderson A, York JD, DiBiase L, Vasilopoulos T, Arnaoutakis G, Beaver T, Martin T, Jeng EI. Dysphagia after cardiac surgery: Prevalence, risk factors, and associated outcomes. J Thorac Cardiovasc Surg. 2023 Feb;165(2):737-746. https://pubmed.ncbi.nlm.nih.gov/33814177/
Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, Corris PA, Ensor CR, Gottlieb J, Hachem RR, Lama V, MartinuT, Neil DAH, Singer LG, Snell G, Vos R. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 2019 May;38(5):493-503. doi: 10.1016/j.healun.2019.03.009. Epub 2019 Apr 3. PMID: 30962148. https://pubmed.ncbi.nlm.nih.gov/30962148/
York JD, Colsky J, Croft K, et al. Dysphagia in lung transplant recipients: prevalence, risk factors and health-related outcomes. J Heart Lung Transplant. 2021;40(4):S349. https://pubmed.ncbi.nlm.nih.gov/35662492/
Additional References Post-Lung Transplant:
Chan, W. W., Ahuja, N., Fisichella, P. M., Gavini, S., Rangan, V., & Vela, M. F. (2020). Extraesophageal syndrome of gastroesophageal reflux: relationships with lung disease and transplantation outcome. Annals of the New York Academy of Sciences, 1482(1), 95–105. https://doi.org/10.1111/nyas.14460
Derousseau, T., Chan, W. W., Cangemi, D., Kaza, V., Lo, W. K., & Gavini, S. (2022). Delayed Gastric Emptying in Prelung Transplant Patients Is Associated With Posttransplant Acute Cellular Rejection Independent of Reflux. Journal of clinical gastroenterology, 56(2), e121–e125. https://doi.org/10.1097/MCG.0000000000001502
Hathorn, K. E., Chan, W. W., & Lo, W. K. (2017). Role of gastroesophageal reflux disease in lung transplantation. World journal of transplantation, 7(2), 103–116. https://pubmed.ncbi.nlm.nih.gov/28507913/
Lo, W. K., Burakoff, R., Goldberg, H. J., Feldman, N., & Chan, W. W. (2015). Pre-transplant impedance measures of reflux are associated with early allograft injury after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation, 34(1), 26–35. https://doi.org/10.1016/j.healun.2014.09.005
Marvin, S., Thibeault, S., Ehlenbach, W.J. (2019). Post-extubation dysphagia: Does timing of evaluation matter? Dysphagia, 34(2), 210–219. https://pubmed.ncbi.nlm.nih.gov/30043081/
Wong, D., & Chan, W. W. (2021). Foregut Dysmotility in the Lung Transplant Patient. Current gastroenterology reports, 23(12), 23. https://doi.org/10.1007/s11894-021-00824-3

